Internet-delivered acceptance and commitment therapy (iACT) for chronic pain—feasibility and preliminary effects in clinical and self-referred patients
Introduction
Around 20% of the general population suffer from chronic pain (1), which commonly affects many areas of life as it tends to interfere with mood, sleep, physical activities, social relations and working life (1-3). Medical and pharmacological treatments are often insufficient in reducing symptoms or disability (4), and there is today a wide consensus regarding the importance of behavioral interventions (5,6). ACT—acceptance and commitment therapy—is a development within the cognitive behavioral tradition where the main treatment objective is to improve functioning and quality of life (QoL) through increased psychological flexibility, defined as the ability to act in alignment with values and long-term goals in the presence of inner discomfort such as pain and distress (7,8). Psychological flexibility is promoted through change processes such as acceptance, cognitive defusion, self-as-context (the skill to observe your inner experiences, not identify with the content of them), present-moment-awareness, values and committed action (9).
ACT has been shown to elevate function and QoL for patients with chronic pain in multiple trials (10-15), however accessibility to this treatment is generally considered to be low. Factors such as number of treatment providers and patients’ personal accessibility issues (such as to take time off work or travel limitations) contribute to the fact that ACT treatment access and reach are limited. For several health conditions internet-delivered treatments have been shown to be efficacious to increase access and reach (16-18). Internet-delivered treatments have also been shown to be cost-effective (19) and time efficient (20), both for therapists and patients for example with regards to travel time and work absence (17,18). Internet-delivered treatment may also facilitate learning and retention as patients can access treatment content at their convenience (17). It can also increase therapists’ ability to monitor and provide timely support to patients throughout an intervention (17).
However, studies of internet-delivered treatments have mostly included patients with well-defined single conditions (17). In contrast, chronic pain patients commonly do not suffer from a single, well-defined diagnosis. Instead chronic pain is often complex with co-occurring: insomnia, depression or anxiety resulting in lowered QoL for many individuals (1,21). Such comorbidities—along with pain, pain interference and/or disability—are important targets for chronic pain treatment (2). Internet-delivered ACT programs (iACT) for chronic pain have previously been evaluated for clinical populations (22,23) as well as with self-referred samples (24-26) with consistently promising effects on pain interference and acceptance but with mixed findings for pain intensity, anxiety and depression, and no positive findings for QoL, insomnia or values (22-26). There have also been discussions whether treatment results are generalizable between clinical and self-referred patients, as self-referred patient groups in web-based treatment studies tend to have higher education and perhaps a less severe condition than clinical populations (17). Internet-delivered treatment often also put demands on patients concerning attention, sitting still and understanding large amounts of information—tasks known to be difficult for many chronic pain patients (1,4,27).
The complexity and vast individual variation between patients with chronic pain calls for a user-centered development to create a design and structure of a digital intervention that takes these challenges into account. In this study, the purpose was to optimize feasibility and treatment effect of an internet-delivered treatment program; iACT, before conducting a subsequent randomized efficacy trial. This implies continuously iterating and evaluating the iACT treatment to meet the needs of both clinical and self-referred chronic pain patients as well as to possibly address some of the common comorbidities by targeting psychological flexibility that may be a common feature for several comorbidities (28,29). Iterations were made based on user insights and acceptability feedback.
The aims of the study were to investigate and compare the following in a clinical and a self-referred sample:
- Evaluate feasibility with regards to acceptability (user insights, comprehensiveness, workability, credibility and adverse events), practicality and usage;
- Evaluate preliminary effects on pain interference (primary outcome), psychological inflexibility, value orientation (process outcomes), QoL, pain intensity, anxiety, insomnia and depressive symptoms (secondary outcomes);
- Evaluate potential treatment mechanisms on the primary outcome (pain interference).
Methods
Design and procedure
The study was conducted at a tertiary pain clinic and was an open pilot and feasibility study with two samples, one clinical and one self-referred. The clinical sample was recruited from the pain clinic between October 10 in 2015 and January 15 in 2017. These participants initiated treatment in five small consecutive cohorts between January 2016 and January 2017. The self-referred sample was recruited via ads in social media between September 23 and October 19, 2016 and started treatment in January 2017 (as one cohort). Inclusion criteria in both samples were persisting (continuous or recurrent) pain for more than 6 months, 18 to 65 years of age, ability to read and write in Swedish, access to a computer with internet, a personal cell phone and time to engage in treatment 20 minutes a day during treatment. Exclusion criteria were planned changes in pain medication, other planned medical interventions aiming at reducing pain, other cognitive behavioral therapy (CBT)/ACT interventions for the past 3 months at study start, and comorbid psychiatric or medical problems that required other care to be prioritized. Before inclusion participants were interviewed by a clinical psychologist specialized in pain. The interview contained a modified version of Mini International Neuropsychiatric Interview (using sections A–B, D–J, L–N) (30), as well as semi-structured questions on pain interference and pain related behavior. For the clinical sample this was done face-to-face, and for the self-referred sample via telephone. Participants were not reimbursed for participation in the study.
Ethics
The study was performed in line with the Helsinki declaration, and was approved by the Regional Ethics Committee in Stockholm (2015/1638-31/2). All participants provided written informed consent.
Inclusion
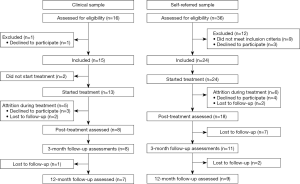
In total 52 patients were assessed for eligibility, of which 39 were included and 37 engaged in treatment. The clinical sample consisted of 15 patients and the self-referred sample of 24 patients, see Figure 1.
Intervention
Treatment was delivered through a desktop-friendly secure digital platform. The intervention (iACT) was based on a face-to-face ACT treatment program developed at a tertiary pain clinic and evaluated in several clinical trials on outcome (14,31,32) and change processes (33,34). A micro-learning format with brief daily content and exercises, use of ordinary (non-medical and non-psychological) language was used to promote experiential learning and workability of content.
The treatment was a 10-week program organized into 40 specific episodes with four episodes to be completed every week. Each episode was intended to take no more than 15 minutes to complete and consisted of a short motivating theory/instruction section, followed by a practical/experiential exercise. Patients gained access to 1 week’s content at a time and were given access to next week’s content upon completion. Each week included one episode with behavior analysis/exposure, one with defusion/acceptance, one with educational content/value work and each week’s program ended with a present-moment-awareness exercise. This combination of micro-content delivery and micro interactions was used to enable participants to learn ACT without information overload (35) as well as to increase personal control and ownership of the learning process (36,37). Content was also built to prompt participants to daily exercise, as the practice and mastery of skills learned in therapy has repeatedly shown to be important for producing positive treatment outcomes (38-40). Content was continuously edited in accordance with patient feedback and suggestions.
During treatment, patients were randomized to receive guidance and support from one of three psychologists trained in CBT and ACT principles (one had 5 years of experience, one had 1 year of experience and one was newly graduated). Supervision meetings were held weekly during recruitment and treatment periods. Therapist-patient communication was largely text-based through a secure messaging system within the treatment platform, and phone support was readily available upon request by the patient or initiated by the therapist due to low patient activity. Therapist support was scheduled for 12 weeks, giving patients a 2-week buffer to handle obstacles to treatment, for example sickness or travel.
Measures
Data were mainly collected through self-report on the secure treatment platform. Data on comorbid psychiatric conditions were collected at the intake interviews.
Background variables
To characterize the two samples, background information on sex, education, occupational status, pain diagnosis type, pain medications, additional symptom burden, age, comorbid psychiatric conditions, body mass index and pain duration in years was collected. Additional symptom burden was self-reported recurring fever, sickness feeling, fatigue, concentration difficulties, memory deficits and sensitivity to stress. All background data were collected before inclusion.
Feasibility variables
Acceptability
Acceptability was assessed by user insights, measuring comprehensiveness, workability, treatment credibility and adverse events. User insights were assessed in writing, by oral feedback and self-report measures. Each treatment week patients were asked for written feedback and answered questions regarding that week’s material. After the treatment period, a convenience sample of 12 patients including both completers and non-completers were invited to provide oral feedback in a semi-structured telephone interview with a psychologist, and eight patients agreed to participate. These patients were asked for feedback regarding time frame, motivation, comprehensiveness, learning, examples, exercises, measures and user-friendliness.
Comprehensiveness and workability were measured with patient ratings from 0 to 10 on each episode. Individual ratings under 7 were used as a prompt for the production team to edit content and mean ratings below 7 were prompts for major editing. Editing was done continuously, and consequently patients included later in the process received a slightly modified version of treatment.
Two episodes were of special interest to the production team (the behavior analysis and the creative hopelessness episodes) as these, when delivered face-to-face, are considered to be very personal, experiential and require extensive therapist guidance. The ratings for these two episodes are described separately in the result section.
Treatment credibility was measured with the credibility rating scale (C-scale) (41). Patients rated how credible, logical, effective and successful they expected the treatment to be in five items on 11-point Likert scales from 0 = Not at all, to 10 = Very much. Patients completed the C-scale at week 5 of the treatment period. In the present study the total score was used to decide satisfactory credibility. Since no established cut off-score exist, a mean score above midpoint (≥25) was considered satisfactory, in line with for example Boersma et al. 2019 (42).
An additional aspect of acceptability was adverse events (43). Patients were continuously prompted to report serious adverse events, such as psychiatric hospitalizations, self-harm and/or suicidal behavior. Clinically meaningful changes in the adverse direction from pre- to post-treatment—calculated as a ≥30% change for pain intensity and 0.5 standard deviation change from baseline assessments of the other outcome variables—were considered deteriorations, in line with recommendations from Dworkin et al. (44).
Practicality
Practicality included number of psychologist minutes per participant, number of messages sent by psychologists per participant, number of requested phone calls and recruitment time. Distance to clinic was calculated as the distance in kilometers between the clinic and patients’ residential postal codes.
Usage
Usage included patient minutes logged in on the platform (this measure was approximate, since a participant was logged out after 20 minutes of inactivity), number of logins and number of sent messages from participants.
Another aspect of usage was compliance, measured in three different areas: content, exposure and values. A patient was considered a completer according to the content criterion if 50% or more of the content was completed within the treatment period, as all of the six psychological flexibility processes then had been introduced (24,45). The criterion for exposure completion was at least one reported exposure. The value completion criterion was at least one formulated personal value.
Preliminary effect evaluation variables
Primary outcome
Pain interference was measured with the Pain Interference Index (PII), a six-item self-report questionnaire assessing the influence of pain on functioning (3). Items are rated on a 7-points Likert scale from 0 = Not at all, to 6 = Completely. Scores range from 0 to 36 and higher scores indicate higher pain interference.
Process variables
Psychological inflexibility was measured with the Psychological Inflexibility in Pain Scale (PIPS) (46). Participants rate twelve items on a 7-point Likert scale from 1 = Never true, to 7 = Always true. The scale has a possible total score ranging from 12 to 84.
Value orientation was measured with the Valuing Questionnaire (VQ) (47,48). The VQ has two scales, value progress and value obstruction, with five items each. Items are rated on a 7-point Likert scale from 0 = Not at all true, to 6 = Completely true. Both scales range from 0 to 30, with higher score on value progress indicating greater behavioral progress towards values and higher scores on value obstruction indicate higher level of behavioral obstruction towards values (48).
Secondary outcome variables
QoL was measured with the Short Form-12 items (SF-12) (49). Items are rated on different scales, for example 1 = excellent, to 5 = poor and 1 = Yes, limited a lot, to 3 = No, not limited at all. Answers are transformed into two composite scores—physical QoL and mental QoL—according to standard US norms as recommended by Gandek et al. (49). The composite scores have a mean of 50, and a standard deviation of 10, meaning that a score of 30 is two standard deviations under the mean, and that 97.5% of the population is expected to have a higher score. The normed scores are not age adjusted and the physical QoL composite score tends to decline with age, while the mental QoL composite score tends to increase slightly with age (50).
Current pain intensity was measured on a numeric rating scale (NRS) ranging from 0 to 10.
Anxiety was measured with the anxiety subscale of Hospital Anxiety and Depression Scale (HADS) (51). Answers on seven symptom/health statements are graded on 4-point Likert scales with varying options, for example 0 = Not at all, and 3 = Most of the time. Scores range from 0 to 21 and a score of 11 or above is recommended as cut-off for probable anxiety disorder (51).
Insomnia was measured with the Insomnia Severity Index (ISI), a seven-item questionnaire with Likert-type scoring options ranging from 0 = Not at all, to 4 = Very much (52). Total scores range from 0 to 28, with higher scores indicating more severe insomnia problems.
Depressive symptoms were measured with the depression subscale of HADS (51). Seven symptom/health statements are graded on Likert scales with varying answers, for example 0 = Not at all, and 3 = Most of the time. Possible scores range from 0 to 21, and scores of 11 or above are recommended as cut-off for probable depression disorder (51).
All effect measures were distributed at baseline, mid-treatment, post-treatment, as well as 3- and 12-month follow-up. Pain interference, psychological inflexibility and current pain intensity were also measured weekly during treatment.
Data analytic approach
All data were analyzed using Stata version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Significance level for all analyses was set to 0.05.
Feasibility
Feasibility data are presented descriptively. Chi square and Student’s t-test were used for comparisons between the clinical and self-referred samples where applicable. Usage data were skewed due to very large variations between participants and therefore medians and interquartile ranges were used. The Mann-Whitney U-test was used to test if usage data from the two samples’ distribution could be from the same population.
Preliminary effects
Preliminary effects were analyzed using multilevel linear modeling.
Little’s MCAR test was used to ascertain that data were missing at random. Data from all included patients were analyzed with an intention-to-treat approach using full information maximum likelihood estimation, where missing values are estimated within the model. Assumptions for multilevel linear modeling were tested and met. Linear mixed-effects model for repeated measures was used to estimate slopes and predict means. Piecewise linear functions in two time periods were estimated: pre- to post-treatment, and post-treatment to 12-month follow-up. Random intercepts, random slopes, and unstructured covariance structure between repeated measurements were included in all models based on goodness of fit measures.
Effect sizes were calculated as Cohen’s d (53) adapted for multilevel linear modeling in accordance with recommendations from Feingold (54). A second set of modeling was done where sample (clinical/self-referred) and time interaction was entered as a covariate to analyze whether the two samples had different slopes or intercept.
Potential process
As a preliminary investigation of potential treatment mechanisms, psychological inflexibility, value progress, value obstruction and pain intensity were entered as covariates in four separate mixed models with the primary outcome pain interference. Linear mixed-effects model for repeated measures was used to estimate slopes based on the weekly measures from pre- to post-treatment. The models included random intercepts, random slopes and unstructured covariance structure.
Results
Participant characteristics
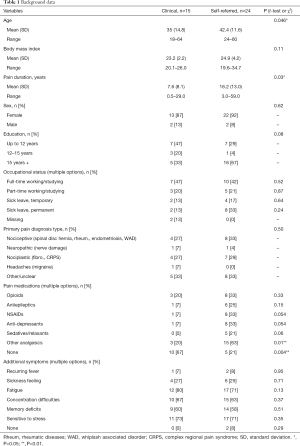
Both samples were predominately female (clinical 87% and self-referred 92%) and displayed a variety of pain diagnoses, see Table 1. The clinical sample was significantly younger with a mean age of 35 and a significantly shorter mean pain duration of 7.6 years while the self-referred sample had a mean age of 42.4 years (P=0.046) and a mean pain duration of 16.2 years (P=0.03), see Table 1 for details.
Full table
In the clinical sample one third had a university degree and in the self-referred sample two thirds had a university degree. In the clinical sample 13% were on permanent sick leave while 33% were on permanent sick leave in the self-referred sample. These differences were not significant, indicating that the groups were not actually different from each other in these aspects and that the observed differences were coincidental.
Of the clinical patients 33% regularly used analgesics while this number was 79% in the self-referred sample (P=0.004). All but two participants experienced additional symptom burden, see Table 1 for complete background data.
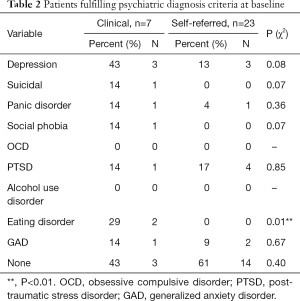
Due to logistic reasons, psychologists’ interview data were missing for nine patients, of which eight were in the clinical sample. However, existing data indicate that there were no differences in rates of psychiatric diagnoses between the clinical and self-referred sample in all diagnoses but one: two clinical sample patients fulfilled diagnostic criteria for bulimia, and none from the self-referred sample. See Table 2 for detailed results.
Full table
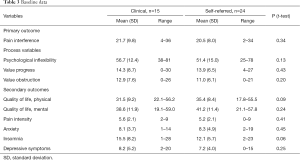
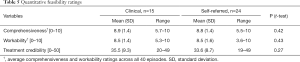
On all outcome variables there were no significant baseline differences between the clinical and the self-referred sample. Both samples displayed on average high pain interference, high psychological inflexibility, QoL below average and moderate pain intensity, see Table 3.
Full table
Feasibility results
Acceptability
Both clinical and self-referred patients’ feedback guided changes in content and structure consecutively. A total of 197 written feedback comments were collected, the largest number for the first week’s content (n=36) and the smallest number for the final week’s material (n=6). The feedback concerned for example spelling and language errors, that a section was hard to grasp, liking or disliking an exercise, suggestions for restructuring material and pointing out especially challenging episodes or exercises. The micro-learning structure was appreciated, and many patients wished for even shorter material. Daily experiential exercises were approved at large, with some patients describing trouble achieving this in practice. Measures were perceived as somewhat exhausting and the interface as difficult to navigate. Patients acknowledged language as easy to understand and instructions to be clear. More detailed patient feedback can be found in the supplementary material (Table S1).
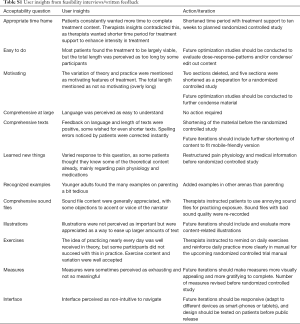
Full table
Comprehensiveness, workability and credibility
Comprehensiveness and workability of treatment content was on average rated above eight (possible range, 0–10) by both samples and no significant differences between samples were found. See Table 4 for details.
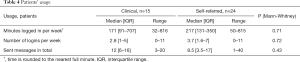
Full table
In total 24 out of 40 episodes received at least one comprehensiveness rating below seven, prompting minor editing. Concerning workability, 29 episodes received at least one rating below seven, requiring minor editing to improve workability.
Three episodes received mean ratings below seven on workability when nine of the clinical participants had completed treatment, prompting major editing to improve workability for the remaining participants. These episodes were on average rated above eight after the revisions. No episodes were on average rated below seven regarding comprehensiveness.
The behavior analysis episode received mean comprehensiveness and workability scores above eight in both samples. The creative hopelessness episode received a comprehensiveness mean score above 8 from both samples, and a workability score of 7.8 in the first four clinical cohorts. This was considered a cause for minor editing and also a change in structure (the episode was moved to an earlier stage in treatment) before the remaining patients (three clinical patients and all self-referred participants) entered treatment, where the episode was rated 8.9 on average.
Treatment credibility data can be found in Table 5. In the clinical sample 80% (n=12) rated the treatment as credible (>25) while 63% (n=15) in the self-referred sample rated treatment as credible. This difference was not significant (χ2=1.68, P=0.43).
Full table
Adverse events
No serious adverse events (psychiatric hospitalizations, self-harm, suicidal behavior) were reported during the study. However, deteriorations as outlined by Dworkin et al. (44) were reported by 13 patients at post-treatment. Five of these patients deteriorated on four or more measures. Deteriorations were attributed to the treatment content (exposure and exercises triggering increased pain and anxiety) by two patients (although still reporting a high degree of satisfaction with the treatment), one attributed the deterioration to a personal loss during the last week of treatment, and the two remaining patients reported that deterioration was due to changes in life circumstances (personal conflicts, new job and stress). At follow-up assessments one of the five multiple deterioration patients had improved on all outcome measures, two patients improved on several measures, and two were lost to follow-up.
Seven of the 13 patients deteriorated on a single outcome variable (of nine) while they at the same time improved on two measures (one patient), four (one patient), five (one patient), seven (one patient) or eight measures (three patients). One patient deteriorated on two measures (anxiety and depressive symptoms), but this patient was still satisfied with treatment and attributed the deterioration to living in an unhealthy relationship. At 12-month follow-up this patient had improved on depressive symptoms, but not on anxiety. No differences were found between clinical and self-referred patients regarding adverse events.
Of the seven patients who withdrew during treatment period, one reported increased pain, three stated stress and three reported content did not match their expectations as their reasons for withdrawal. The reasons for withdrawal for the four additional patients that were lost to follow-up during treatment are unknown.
Practicality
Psychologists used on average 13.5 minutes per patient per week in the clinical sample and this was significantly more than the 8 minutes in the self-referred sample. Very few patients requested telephone contact during treatment, and there were no phone calls in the clinical sample and an average of 0.2 per patient in the self-referred sample, see Table 6.
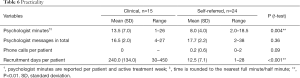
Full table
Total recruitment time was 15 months for the 15 patients in the clinical sample and 1 month for the 24 patients for the self-referred sample (P<0.001). Distance to the clinic was in median 40 kilometer (IQR, 9–161) for the clinical sample and 426 km (IQR, 211–577) for the self-referred sample (P<0.001).
Usage
There were no significant differences in usage between clinical and self-referred patients, see Table 4 for details.
Completion
According to the study’s pre-defined criteria of completion 60–80% of the clinical patients were considered completers while a consistent 58% of the self-referred patients were completers across criteria, see Table 7. The differences between samples were not significant.

Full table
No significant differences were found in number of completed episodes between samples. Both clinical and self-referred patients reported around nine completed exposures on average and wrote down over twelve values, see Table 8 for details.

Full table
Preliminary effects
Results from the linear mixed effects models revealed significant improvements on pain interference, psychological inflexibility, value progress, value obstruction, QoL, depressive symptoms, pain intensity, anxiety and insomnia from pre- to post-treatment. Effect size on the primary outcome pain interference was medium, effects sizes on process variables were large for psychological inflexibility, medium for value progress and varied from small to medium for secondary outcomes value obstruction, QoL, insomnia, depressive symptoms, pain intensity and anxiety, see Table 9 for details.
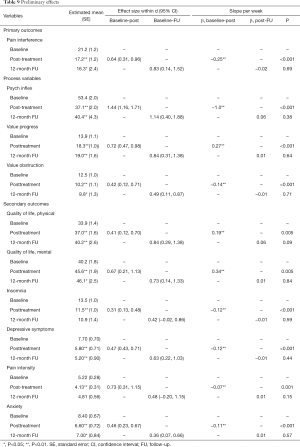
Full table
No significant effect of time was found from post-treatment to 3- or 12-month follow-up for any of the outcome variables, indicating stability of improvements, see Table 9.
For the analyses adding an interaction term for time × sample, there were no significant interaction effect, indicating no differences in estimates between clinical and self-referred participants’ intercept or slope in any of the outcomes. See Figures 2,3 for a graphic presentation of primary outcome pain interference and primary process measure psychological flexibility from baseline to 12-month follow-up.
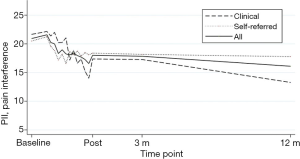
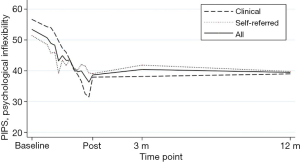
Potential process
The potential process variables were all found to have significant beta coefficients for the primary outcome pain interference when entered as covariates in mixed model analyses from pre- to post-treatment. The effect of time was no longer significant when value progress was entered into the model, and the effect of time became reversed (pain interference increased with time) when psychological inflexibility was entered into the model, indicating a significant influence of these process variables on the improvements in pain interference. See Table 10 for details. When sample was entered as a covariate, it did not have a significant impact in any of the models.

Full table
Discussion
The aim of the present study was to evaluate feasibility as well as preliminary treatment effects and change mechanisms of iACT, an iACT treatment, in two samples of adult patients with complex chronic pain. The results suggest that the micro-learning format of the treatment was feasible for both samples. The preliminary effect evaluation rendered positive effects on all outcomes for both samples, and the preliminary mechanism analyses indicated that psychological inflexibility and value progress had significant influence on pain interference.
Participant insights provided valuable information on how treatment content and delivery can be optimized to further increase compliance, retention and possibly treatment effect. The similar ratings on comprehensiveness, workability and credibility between samples indicate that iACT is suitable for both clinical and self-referred patients. Both clinical and self-referred patients managed to complete the episodes behavior analysis and creative hopelessness on their own, indicating that the transition from face-to-face to internet-delivery can be done also with complex, experiential treatment content. The majority of both self-referred and clinical patients formulated a range of values and performed exposures on their own. Completion rates were similar to what has been reported in previous studies on iACT for chronic pain (22-24,26) but leaves room for improvements. On average, patients completed around 60% of treatment content, implying that the treatment program may be condensed without negative effects on treatment outcome. Future studies should aim at optimizing treatment content itself, as well as the length of treatment, in order to minimize redundant content. Also, investigations on dose-response-relationships and the benefits and pitfalls of providing participants with extensive or possibly excessive information, are important questions that should be addressed empirically. The utility of modifying the intervention to meet different needs, such as audio-delivery of content for persons who prefer listening above reading, could also be investigated.
No serious adverse events were reported. However, 13 patients reported deteriorations. Three of these patients experienced increased pain, which is an expected side-effect for some patients, since the treatment encourages engagement in potentially painful activities that previously have been avoided. It should also be noted that eight of these 13 patients deteriorated in only one or two out of nine outcomes while they improved on between two and eight of the others. Three patients that deteriorated on multiple outcomes attributed the causes for deterioration to other factors than treatment, while two multiple deterioration patients attributed deteriorations to the treatment. Based on the lack of serious adverse events, the preliminary results suggest that the intervention is safe, even though some patients may experience deteriorations.
Adding to the evidence-base for the cost-effectiveness of internet-delivered treatments, the psychologists in the study spent less than 15 minutes a week on their patients, while patients spent on average more than 2 hours working with the treatment each week. Notably, patients spent more time interacting with treatment content and exercises than usually spent with a therapist in a standard face-to-face intervention without a need for the patient to be absent from work, and without any implications for treatment integrity or effects (17,18).
The results were obtained with two diverse samples of patients suffering from complex chronic pain conditions, including young adults as well as individuals around retirement age. The clinical sample had an average pain duration of nearly 8 years, and the self-referred sample just over 16 years. Almost all patients suffered from comorbidities, with fatigue and concentration deficits being the most common. A large part of patients was on sick leave (clinical sample 26%, self-referred sample 50%) and had one or more psychiatric diagnosis at baseline (clinical 58%, self-referred 39%). Both samples thus displayed complex symptoms and high pain interference at baseline, with similar levels of symptom burden in outcome measures at baseline, post-treatment and follow-up. The results suggest that the differences between clinical and self-referred patients may not be crucial to treatment engagement and effects. Patients usage data showed that it was feasible to prompt patients in both samples to log in and engage in treatment several times a week, despite the common challenges that these patients face (1,27).
Recruitment rate was one participant per month for 15 months in the clinical sample and 24 participants in 1 month in self-referral. Geographical reach was 40 km (median) for the clinical patients and 400 km (median) for self-referred patients, adding to previous research illustrating the utility of internet-delivery to increase reach and access with satisfactory treatment effects (55,56).
With improvements in pain interference, psychological flexibility, value orientation, QoL, insomnia, pain intensity, depressive symptoms and anxiety during treatment and up to 1-year follow-up, iACT may be beneficial in areas—such as values, QoL and insomnia—that previous internet-ACT programs have not been successful (22-26).
Future studies should include large randomized controlled trials to evaluate efficacy as well as moderators and mediators of treatment effects. Health economic implications or cost-benefit analyses are of interest, since high health care costs and sick leave is common among chronic pain patients. Furthermore, it could also be important to keep up with the increased smartphone use habits (57) and a smartphone friendly version might increase compliance and treatment effect as it provides opportunities for real-time assessments and just-in-time interaction during an intervention (17).
The preliminary analyses of treatment mechanism indicate that psychological inflexibility and value progress may function as mediators of treatment effects and therefore constitute potential treatment targets. This is in line with previous analyses of mechanisms in ACT treatments (58). The change from minus to plus on the effect of time on pain interference when psychological inflexibility was entered into the model makes the interpretation more unclear, but according to both Kenny et al. and MacKinnon et al. (59,60) this change in direction of effect can also be a sign of inconsistent mediation or a negative suppressor variable. This needs to be examined further in larger samples.
The results are obtained with two small samples receiving slightly modified versions of content and should therefore be interpreted with caution. Comparisons between the clinical and self-referred samples could possibly be confounded by differences in timing of recruitment, treatment design and structure. Attrition increased over time, which makes the follow-up results less reliable. It is possible that participation in the study provided a considerable challenge for included patients, and that this negatively influenced attrition. In addition to engaging in treatment, participants were asked to repeatedly give written and oral feedback and complete a large number of both feasibility and effect measures (perceived as less meaningful to the patients, especially as they themselves could not follow the progress) at numerous occasions. Lastly, since both samples consisted of patients who volunteered for an internet-delivered treatment, the results may not be generalized to all chronic pain patients.
Conclusions
The feasibility evaluation suggests that iACT is feasible for both clinical and self-referred chronic pain patients. The preliminary positive effects on pain interference, psychological inflexibility and QoL motivate further large-scale randomized efficacy trials.
Acknowledgments
The authors wish to thank all patients for their time and effort to participate. We also thank Dr Martin Jonsjö for theoretical and statistical support. This study was partially funded by AFA Insurance and the regional agreement on medical training and clinical research between Stockholm City Council and Karolinska Institutet. Funders had no influence on design, data collection, analyses or manuscript.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/mhealth.2020.02.02). EA serves as the unpaid editorial board member of mHealth from Dec 2018 to Nov 2020. JR reports grants from AFA Insurance, during the conduct of the study. EA reports personal fees from Royalties from a book on health anxiety, outside the submitted work. BL reports other from Dahlia Behandlingsutvärdering AB, other from Pear Therapeutics Inc, outside the submitted work. RKW reports grants from AFA Insurance, grants from ALF grant provided by the Stockholm County Council, during the conduct of the study; grants from Clinical research appointment provided by the Stockholm County Council, outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was performed in line with the Helsinki declaration, and was approved by the Regional Ethics Committee in Stockholm (2015/1638-31/2). All participants provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287-333. [Crossref] [PubMed]
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19. [Crossref] [PubMed]
- Kemani MK, Zetterqvist V, Kanstrup M, et al. A validation of the pain interference index in adults with longstanding pain. Acta Anaesthesiol Scand 2016;60:250-8. [Crossref] [PubMed]
- Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Can J Psychiatry 2008;53:224-34. [Crossref] [PubMed]
- Eccleston C. Role of psychology in pain management. Br J Anaesth 2001;87:144-52. [Crossref] [PubMed]
- Morley S, Williams A. New Developments in the Psychological Management of Chronic Pain. Can J Psychiatry 2015;60:168-75. [Crossref] [PubMed]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: An Experiential Approach to Behavior Change. New York: Guilford Publications, 1999.
- Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther 2006;44:1-25. [Crossref] [PubMed]
- Hayes SC, Pistorello J, Levin ME. Acceptance and commitment therapy as a unified model of behavior change. Couns Psychol 2012;40:976-1002. [Crossref]
- Simpson PA, Mars T, Esteves JE. A systematic review of randomised controlled trials using Acceptance and commitment therapy as an intervention in the management of non-malignant, chronic pain in adults. Int J Osteopath Med 2017;24:18-31. [Crossref]
- Vowles KE, McCracken LM. Acceptance and values-based action in chronic pain: a study of treatment effectiveness and process. J Consult Clin Psychol 2008;76:397-407. [Crossref] [PubMed]
- Vowles KE, McCracken LM, O'Brien JZ. Acceptance and values-based action in chronic pain: A three-year follow-up analysis of treatment effectiveness and process. Behav Res Ther 2011;49:748-55. [Crossref] [PubMed]
- Wetherell JL, Afari N, Rutledge T, et al. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain 2011;152:2098-107. [Crossref] [PubMed]
- Wicksell RK, Kemani M, Jensen K, et al. Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial. Eur J Pain 2013;17:599-611. [Crossref] [PubMed]
- Wicksell RK, Melin L, Lekander M, et al. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain – A randomized controlled trial. Pain 2009;141:248-57. [Crossref] [PubMed]
- Hedman E, Carlbring P, Ljótsson B, et al. Internetbaserad psykologisk behandling: evidens, indikation och praktiskt genomförande. Stockholm: Natur & Kultur, 2014.
- Andersson G, Titov N. Advantages and limitations of Internet-based interventions for common mental disorders. World Psychiatry 2014;13:4-11. [Crossref] [PubMed]
- Buhrman M, Gordh T, Andersson G. Internet interventions for chronic pain including headache: A systematic review. Internet Interv 2016;4:17-34. [Crossref] [PubMed]
- Hedman E, Ljótsson B, Lindefors N. Cognitive behavior therapy via the Internet: a systematic review of applications, clinical efficacy and cost-effectiveness. Expert Rev Pharmacoecon Outcomes Res 2012;12:745-64. [Crossref] [PubMed]
- Andrews G, Davies M, Titov N. Effectiveness randomized controlled trial of face to face versus Internet cognitive behaviour therapy for social phobia. Aust N Z J Psychiatry 2011;45:337-40. [Crossref] [PubMed]
- McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain 2008;9:771-83. [Crossref] [PubMed]
- Scott W, Chilcot J, Guildford B, et al. Feasibility randomized-controlled trial of online Acceptance and Commitment Therapy for patients with complex chronic pain in the United Kingdom. Eur J Pain 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Buhrman M, Skoglund A, Husell J, et al. Guided internet-delivered acceptance and commitment therapy for chronic pain patients: a randomized controlled trial. Behav Res Ther 2013;51:307-15. [Crossref] [PubMed]
- Trompetter HR, Bohlmeijer ET, Veehof MM, et al. Internet-based guided self-help intervention for chronic pain based on Acceptance and Commitment Therapy: A randomized controlled trial. J Behav Med 2015;38:66-80. [Crossref] [PubMed]
- Simister HD, Tkachuk GA, Shay BL, et al. Randomized Controlled Trial of Online Acceptance and Commitment Therapy for Fibromyalgia. J Pain 2018;19:741-53. [Crossref] [PubMed]
- Lin J, Paganini S, Sander L, et al. An Internet-Based Intervention for Chronic Pain. Dtsch Arztebl Int 2017;114:681-8. [PubMed]
- Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 1999;125:356-66. [Crossref] [PubMed]
- Vowles KE, McCracken LM. Comparing the role of psychological flexibility and traditional pain management coping strategies in chronic pain treatment outcomes. Behav Res Ther 2010;48:141-6. [Crossref] [PubMed]
- McCracken LM, Morley S. The Psychological Flexibility Model: A Basis for Integration and Progress in Psychological Approaches to Chronic Pain Management. J Pain 2014;15:221-34. [Crossref] [PubMed]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59 Suppl 20:22-33. [PubMed]
- Wicksell RK, Ahlqvist J, Bring A, et al. Can exposure and acceptance strategies improve functioning and life satisfaction in people with chronic pain and whiplash-associated disorders (WAD)? A randomized controlled trial. Cogn Behav Ther 2008;37:169-82. [Crossref] [PubMed]
- Wicksell RK, Melin L, Lekander M, et al. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain – A randomized controlled trial. Pain 2009;141:248-57. [Crossref] [PubMed]
- Wicksell RK, Olsson GL, Hayes SC. Mediators of change in Acceptance and Commitment Therapy for pediatric chronic pain. Pain 2011;152:2792-801. [Crossref] [PubMed]
- Wicksell RK, Olsson GL, Hayes SC. Psychological flexibility as a mediator of improvement in Acceptance and Commitment Therapy for patients with chronic pain following whiplash. Eur J Pain 2010;14:1059.e1-1059.e11. [Crossref] [PubMed]
- Bruck PA, Motiwalla L, Foerster F. editors. Mobile Learning with Micro-content: A Framework and Evaluation. Bled eConference, 2012.
- Wong LH. A learner‐centric view of mobile seamless learning. Br J Educ Technol 2012;43:E19-23. [Crossref]
- Ally M. Mobile learning: Transforming the delivery of education and training. Athabasca University Press, 2009.
- Mausbach BT, Moore R, Roesch S, et al. The relationship between homework compliance and therapy outcomes: An updated meta-analysis. Cognit Ther Res 2010;34:429-38. [Crossref] [PubMed]
- Kazantzis N, Lampropoulos GK. Reflecting on homework in psychotherapy: What can we conclude from research and experience? J Clin Psychol 2002;58:577-85. [Crossref] [PubMed]
- Kazantzis N, Deane FP, Ronan KR. Homework assignments in cognitive and behavioral therapy: A meta‐analysis. Clinical Psychology: Science and Practice 2000;7:189-202. [Crossref]
- Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry 1972;3:257-60. [Crossref]
- Boersma K, Södermark M, Hesser H, et al. Efficacy of a transdiagnostic emotion–focused exposure treatment for chronic pain patients with comorbid anxiety and depression. Pain 2019;160:1708-18. [Crossref] [PubMed]
- Rozental A, Andersson G, Boettcher J, et al. Consensus statement on defining and measuring negative effects of Internet interventions. Internet Interventions 2014;1:12-9. [Crossref]
- Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9:105-21. [Crossref] [PubMed]
- Hedman E, Axelsson E, Gorling A, et al. Internet-delivered exposure-based cognitive-behavioural therapy and behavioural stress management for severe health anxiety: randomised controlled trial. Br J Psychiatry 2014;205:307-14. [Crossref] [PubMed]
- Wicksell RK, Renofalt J, Olsson GL, et al. Avoidance and cognitive fusion--central components in pain related disability? Development and preliminary validation of the Psychological Inflexibility in Pain Scale (PIPS). Eur J Pain 2008;12:491-500. [Crossref] [PubMed]
- Smout M, Davies M, Burns N, et al. Development of the Valuing Questionnaire (VQ). J Contextual Behav Sci 2014;3:164-72. [Crossref]
- Rickardsson J, Zetterqvist V, Kemani MK, et al. Assessing values – Psychometric properties of the Swedish version of the Valuing Questionnaire in adults with chronic pain. J Contextual Behav Sci 2019;14:40-9. [Crossref]
- Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:1171-8. [Crossref] [PubMed]
- Ware JE Jr., Kosinski M, Keller SD. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, Massachusetts. The Health Institute, New England Medical Center; 1995.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [Crossref] [PubMed]
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2:297-307. [Crossref] [PubMed]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. Hillsdale: L. Erlbaum Associates, 1988.
- Feingold A. Effect sizes for growth-modeling analysis for controlled clinical trials in the same metric as for classical analysis. Psychol Methods 2009;14:43-53. [Crossref] [PubMed]
- Enander J, Andersson E, Mataix-Cols D, et al. Therapist guided internet based cognitive behavioural therapy for body dysmorphic disorder: single blind randomised controlled trial. BMJ 2016;352:i241. [Crossref] [PubMed]
- Andrews G, Basu A, Cuijpers P, et al. Computer therapy for the anxiety and depression disorders is effective, acceptable and practical health care: An updated meta-analysis. J Anxiety Disord 2018;55:70-8. [Crossref] [PubMed]
- Clement J. Mobile internet traffic as percentage of total web traffic in August 2019, by region Statista.com: Statista; 2019 [updated September 9 2019. Available online: https://www.statista.com/statistics/306528/share-of-mobile-internet-traffic-in-global-regions/
- Stockton D, Kellett S, Berrios R, et al. Identifying the Underlying Mechanisms of Change During Acceptance and Commitment Therapy (ACT): A Systematic Review of Contemporary Mediation Studies. Behav Cogn Psychother 2019;47:332-62. [Crossref] [PubMed]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci 2000;1:173-81. [Crossref] [PubMed]
- Kenny DA. Mediation: David Kenny; 2018 [updated September 25, 2018]. Available online: http://davidakenny.net/cm/mediate.htm
Cite this article as: Rickardsson J, Zetterqvist V, Gentili C, Andersson E, Holmström L, Lekander M, Persson M, Persson J, Ljótsson B, Wicksell RK. Internet-delivered acceptance and commitment therapy (iACT) for chronic pain—feasibility and preliminary effects in clinical and self-referred patients. mHealth 2020;6:27.