-
PDF
- Split View
-
Views
-
Cite
Cite
Naykky Singh-Ospina, Spyridoula Maraka, Rene Rodriguez-Gutierrez, Caroline Davidge-Pitts, Todd B Nippoldt, Larry J Prokop, Mohammad Hassan Murad, Effect of Sex Steroids on the Bone Health of Transgender Individuals: A Systematic Review and Meta-Analysis, The Journal of Clinical Endocrinology & Metabolism, Volume 102, Issue 11, 1 November 2017, Pages 3904–3913, https://doi.org/10.1210/jc.2017-01642
Close - Share Icon Share
Abstract
The impact of sex steroids on bone health in transgender individuals is unclear.
A comprehensive search of several databases to 7 April 2015 was conducted for studies evaluating bone health in transgender individuals receiving sex steroids. Pairs of reviewers selected and appraised studies. A random effects model was used to pool weighted mean differences and 95% confidence intervals (CIs).
Thirteen studies evaluating 639 transgender individuals were identified [392 male-to-female (MTF), 247 female-to-male (FTM)]. In FTM individuals and compared with baseline values before initiation of masculinizing hormone therapy, there was no statistically significant difference in the lumbar spine, femoral neck, or total hip bone mineral density (BMD) when assessed at 12 and 24 months. In MTF individuals and compared with baseline values before initiation of feminizing hormone therapy, there was a statistically significant increase in lumbar spine BMD at 12 months (0.04 g/cm2; 95% CI, 0.03 to 0.06 g/cm2) and 24 months (0.06 g/cm2; 95% CI, 0.04 to 0.08 g/cm2). Fracture rates were evaluated in a single cohort of 53 MTF and 53 FTM individuals, with no events at 12 months. The body of evidence is derived mostly from observational studies at moderate risk of bias.
In FTM individuals, masculinizing hormone therapy was not associated with significant changes in BMD, whereas in MTF individuals feminizing hormone therapy was associated with an increase in BMD at the lumbar spine. The impact of these BMD changes on patient-important outcomes such as fracture risk is uncertain.
The precise number of individuals who experience transsexualism during their lifetime is uncertain; however, a prevalence of 4.6 in 100,000 individuals has been estimated (probably an underestimation) (1, 2). The number of transgender individuals who seek medical care appears to be increasing (3). Low-quality evidence (i.e., which translates into low confidence in the balance of risk and benefits) suggests that hormonal intervention to achieve desired secondary sexual characteristics is beneficial in terms of psychological functioning and overall quality of life (4). On the other hand, unfavorable changes in lipid profile, with unclear effects on cardiovascular outcomes, have been reported (5).
Many studies have established sex steroids (estrogen and testosterone) as important regulators of bone health both in men and women (6–8). In men, testosterone plays an important role for male skeletal homeostasis, and a minimal estradiol level is needed for optimal skeletal maturation (7). In women, there is a positive net effect of estrogen action on bone homeostasis, but the effect of androgens is less clear (6, 8). This physiological background suggests that changes in bone health could be expected as a result of sex steroid therapy.
The effect of sex steroids on the bone health of transgender individuals has been scarcely studied, and although clinical reviews about the topic are available, a systematic evaluation and quantification of the effect and potential clinical relevance when related to patient-important outcomes (e.g., fracture risk) is not available (1, 9).
Clinicians taking care of transgender individuals seeking sex steroid treatment need information about the risks and benefits associated with therapy. In the case of bone health, determining the degree and timing of expected changes in bone mineral density (BMD) or the incidence of fractures could help identify the need for follow-up and treatment considerations or provide reassurance if no detrimental effect is to be expected. To this end, we performed a systematic review and meta-analysis of the available evidence assessing the effects of sex steroid treatment on the bone health of transgender individuals.
Methods
We performed a systematic review and meta-analysis to estimate the impact of sex steroid treatment of adolescent and adult transgender individuals on bone health. Outcomes of interest were BMD and the incidence of fractures. This report followed a systematic review protocol developed in collaboration with experts from the Endocrine Society and the current standard for reporting of systematic reviews (10).
Eligibility criteria
We included randomized trials, observational studies, and case series of transsexual individuals who received sex steroids. We included studies evaluating adolescents and adult transgender individuals (gender-confirming surgery was not an exclusion criterion). Eligible studies exposed natal men seeking transition to the female sex [male-to-female (MTF)] to cross-sex hormone therapy including estrogen, antiandrogens (cyproterone acetate, spironolactone), or gonadotropin-releasing hormone (GnRH) agonists and women seeking transition to the male sex [female-to-male (FTM)] to testosterone. We included studies that compared baseline values of BMD to posttherapy values in the same individuals (treatment-naive patients, receiving therapy for ≥3 months) or those that compared BMD values in the transgender group (after ≥3 months of therapy) with a control or reference group. Outcomes of interest included effects on bone health (BMD at the lumbar spine, femoral neck, total hip, and fractures).
We excluded studies in which the required information to determine eligibility was not available in the published manuscript and no response from the authors was obtained. We included studies regardless of their publication status, language, or size. Review articles, commentaries, and letters that did not contain original data were excluded.
Study identification
A comprehensive search of several databases from 1980 to 7 April 2015 was conducted. Databases included Ovid Medline In-Process & Other Non-Indexed Citations, Ovid Medline, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, and Scopus. An experienced librarian (L.J.P.) designed the search strategy with input from study investigators with expertise in conducting systematic reviews. Controlled vocabulary supplemented with keywords was used to search for studies of outcomes of hormone therapy on transgender individuals. The search strategy is available in the Supplemental Appendix. We reviewed the reference list of narrative reviews and consulted with experts to identify additional references.
The search results were uploaded into a systematic review software (DistillerSR, Ottawa, Canada). Reviewers working independently and in duplicate reviewed all abstracts and titles for inclusion (N.S.O., S.M., and R.R.G.). After abstract screening and retrieval of potentially eligible studies, the full-text publications were assessed for eligibility, with excellent chance-adjusted interreviewer agreement (κ statistic = 0.82). Duplicate studies and studies with overlapping populations were excluded. Disagreements were resolved by consensus (the two reviewers discussed the discrepancy and reached a decision).
Data collection and management
Reviewers working independently and in duplicate (N.S.O., S.M., and R.R.G.) using a standardized form collected the following information from each eligible study: baseline clinical features such as age, weight, body mass index, and group (MTF or FTM); proportion of patients with gender confirmation surgery and definition; type of intervention (medication, dose, route, frequency) and duration of the exposure at the time of outcome assessment; and outcomes (BMD, number of fractures). We also extracted the definition of controls used in the applicable studies. Disagreements were resolved by consensus.
Risk of bias assessment
We used the Newcastle Ottawa tool to evaluate the risk of bias in observational studies. This tool evaluates the selection of study cohorts, the comparability of the study cohorts, and the ascertainment of exposure and outcomes (11). Reviewers working independently assessed the risk of bias of included studies in duplicate. Any disagreements were resolved by consensus.
Author contact
To reduce reporting bias, we contacted by e-mail the corresponding authors (or any other author if we were not able to reach the corresponding author) of each of the eligible studies in which clarification or more information was needed to determine eligibility or to complete analyses. We contacted seven authors for clarification and to obtain further data; two replied.
Meta-analysis
We conducted random-effects meta-analysis by using the DerSimonian–Laird random effects method to pool mean differences for continuous outcomes and their associated 95% confidence intervals (CIs) (12). Longitudinal and cross-sectional studies were analyzed separately. We subtracted the baseline BMD value (grams per square centimeter) from the follow-up measurement when calculating the mean differences in BMD. Therefore, negative values indicate a decrease from baseline and positive values an increase. When comparing values against a control group, we subtracted the control group BMD value from the transgender group value, so a positive value indicated higher value for the transgender group and a negative value indicated a lower value for the transgender group. We also estimated the proportion of patients with fractures in the included studies. Inconsistency was assessed with the I2 statistic, with values <25% indicating low and >75% indicating high inconsistency (13). Analysis was conducted in Stata Statistical Software: Release 14 (StataCorp LP, College Station, TX).
Subgroups and sensitivity analyses
We planned to measure the difference in effect sizes between subgroups based on population type (adolescent vs. adults), different treatment regimens (e.g., oral vs. transdermal estrogen, agonist alone vs. combination therapy), and outcome characteristics (e.g., symptomatic vs. asymptomatic fractures). Sensitivity analyses were conducted to explain possible inconsistencies across study results.
Results
Study identification
A total of 391 potentially eligible articles were identified through our systematic database search, of which 13 were ultimately eligible (14–26), after exclusion of studies that represented overlapping populations (27–30). The complete study selection process is described in Supplemental Fig. 1.
Across all studies, 639 transgender patients were included in the analysis. These included 392 MTF individuals (9 studies) and 247 FTM participants (8 studies). Twelve studies evaluated changes in BMD, and only 1 evaluated fracture rates.
A summary of the included studies is found in Table 1. All the included studies were observational. Eleven provided before- and after-treatment comparisons of the same patients and 2 compared the results of transgender individuals with those of controls. Only one study provided information on adolescent populations. The mean age of transgender individuals in the included studies ranged from 19 to 43 years. The treatment regimen of MTF individuals included various doses of oral, transdermal, or intramuscular (IM) estrogens and some regimens included cyproterone acetate, GnRH agonists (goserelin, triptorelin), spironolactone, or anastrozole. Most FTM patients received IM preparations of testosterone and some transdermal or oral testosterone. Outcome assessment was performed at 12 and 24 months in the majority of the studies, although mean follow-up times were provided in 3 studies.
Characteristics of Included Studies
| Study . | Country . | Design . | Comparison Group . | Patients . | No. of Patients . | Mean Age . | Mean BMI . | % of Patients with GCS . | Description of Intervention . | Duration of Exposure . |
|---|---|---|---|---|---|---|---|---|---|---|
| Dittrich et al., 2005 (14) | Germany | Cohort | Same subjects (before and after) | MTF | 60 | 38.37 | 24.19a | 0 | 3.8 mg goserelin every 4 wk and 6 mg oral estradiol-17 valerate per d. | 24 mo |
| Klink et al., 2015 (16) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 15 | 14.9 | 20.3 | 100 | Triptorelin 3.75 mg every 4 wk SC from 11.4–18.3 y. | GnRH 1.3 y (range, 0.5–3.8) and CSH therapy 5.8 y (range, 3.0–8.0) |
| In the age range from 15.6 to 19 y transgender women were prescribed incremental dosing of 17-estradiol. | ||||||||||
| At a minimum age of 18 y, after gonadectomy, GnRH treatment was terminated and CSH therapy continued. | ||||||||||
| Same subjects (before and after) | FTM | 19 | 15.0 | 20.9 | 100 | Testosterone esters (Sustanon 250 mg/mL) every 2–4 wk in incremental dosages. | GnRH 1.5 y (range, 0.25–5.2) and CSH 5.4 y (range, 2.8–7.8) | |||
| At a minimum age of 18 y, after gonadectomy, GnRH treatment was terminated and CSH therapy continued. | ||||||||||
| Mueller et al., 2011 (18) | Germany | Cohort | Same subjects (before and after) | MTF | 84 | 36.3 | 22.3 | 0 | 3.8 mg goserelin every 4 wk and a dosage of 10 mg estradiol 17-valerate IM every 10 d. | 24 mo |
| Mueller et al., 2010 (17) | Germany | Cohort | Same subjects (before and after) | FTM | 45 | 30.4 | 24.1 | 0 | Testosterone undecanoate 1000 mg IM every 12 wk. | 12 and 24 mo |
| Pelusi et al., 2014 (19) | Italy | Cohort | Same subjects (before and after) | FTM | 15 | 30.9 | NA | 0 | Testosterone enanthate IM at a dosage of 100 mg every 10 d (n = 15; TD group). | 12 mo |
| Same subjects (before and after) | FTM | 15 | 29.4 | NA | 0 | Testosterone gel at a dosage of 50 mg/d every evening. | 12 mo | |||
| Same subjects (before and after) | FTM | 15 | 28.2 | NA | 0 | Testosterone undecanoate at a dosage of 1000 mg at wk 0, wk 6, and thereafter, every 12 wk. | 12 mo | |||
| Reutrakul et al., 1998 (20) | Thailand | Cohort | Controls | MTF | 11 | 21.2 | NA | 0 | Estradiol valerate 10 mg per ampule, mestranol 0.05 mg norethisterone 1 mg, or contraceptive pills, ethinyl estradiol, and several doses of levonorgestrel. | <24 mo |
| Controls | MTF | 17 | 24.1 | NA | 0 | NA. | >24 mo | |||
| Sosa et al., 2003 (21) | Spain | Cohort | Controls | MTF | 27 | 43 | 26 | 0 | Contraceptive pills (ethinyl estradiol + cyproterone acetate or levonorgestrel), oral estrogen (conjugated equine), and depot estrogens (estradiol valerate or mestranol + norethisterone). | 201 mo (108)b (range 3–35 y) |
| Turner et al., 2004 (22) | USA | Cohort | Same subjects (before and after) | FTM | 8 | 33.1 | NA | 0 | Testosterone IM (mean dosage of 70.4 ±4.5 mg weekly). | 24 mo |
| Van Caenegem et al., 2015 (24) | Belgium | Cohort | Same subjects (before and after) | FTM | 23 | 27 | 24.5 | NA | Testosterone undecanoate of 1000 mg IM. Injections were administered at baseline, after 6 and 18 wk, and from then once every 12 wk. | 12 mo |
| Van Caenegem et al., 2015 (23) | Belgium | Cohort | Same subjects (before and after) | MTF | 49 | 33 | NA | 0 | Oral estradiol valerate, 4 mg daily or transdermal 17-β estradiol 100 μg/24 h for patients >45 years old or both combined with oral cyproterone acetate 50 mg daily. Transdermal estrogens were used in older transgender women because they carry a lower thromboembolic risk. | 12 and 24 mo |
| van Kesteren et al., 1996 (15) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 56 | 33 | 22 | 0 | Cyproterone acetate 100 mg/d with ethinylestradiol or transdermal estradiol twice a week. | 12 mo |
| FTM | 35 | 25 | 23 | 0 | Testosterone esters IM every 2 wk, Sustanon 250, or testosterone undecanoate 160 mg/d orally. | 12 mo | ||||
| van Kesteren et al., 1998 (25) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 20 | 25.4 | 22.1 | 100 | Cyproterone acetate 100 mg/d in combination with ethinylestradiol 100 μg/d until gonadectomy; after surgery just estrogen to maintain female features. | 45.5 mo (9.7)b (range, 32–63) |
| Same subjects (before and after) | FTM | 19 | 25.0 | 22.1 | 100 | Testosterone esters 250 mg/2 wk; after gonadectomy most patients continued with IM every 2–3 wk. | 38.2 mo (8.6)b (range, 28–53) | |||
| Wierckx et al., 2014 (26)c | Belgium | Cohort | Same subjects (before and after)/no comparison | MTF | 47 | 31.7 | 23.9 | NA | 50 mg cyproterone acetate and 4 mg estradiol valerate daily, whereas those >45 y old received 50 mg CA daily together with 100 μg/24 h transdermal 17-β estradiol. | 12 mo |
| Same subjects (before and after)/no comparison | MTF | 6 | 19.3 | 22.9 | NA | 50 mg cyproterone acetate and 4 mg estradiol valerate daily, whereas those >45 y old received 50 mg CA daily together with 100 μg/24 h transdermal 17-β estradiol. | 12 mo | |||
| Same subjects (before and after)/no comparison | FTM | 27 | 27.3 | 24.5 | NA | Testosterone undecanoate IM every 3 mo. | 12 mo | |||
| Same subjects (before and after)/no comparison | FTM | 26 | 21.7 | 25.2 | NA | Testosterone undecanoate IM every 3 mo. | 12 mo |
| Study . | Country . | Design . | Comparison Group . | Patients . | No. of Patients . | Mean Age . | Mean BMI . | % of Patients with GCS . | Description of Intervention . | Duration of Exposure . |
|---|---|---|---|---|---|---|---|---|---|---|
| Dittrich et al., 2005 (14) | Germany | Cohort | Same subjects (before and after) | MTF | 60 | 38.37 | 24.19a | 0 | 3.8 mg goserelin every 4 wk and 6 mg oral estradiol-17 valerate per d. | 24 mo |
| Klink et al., 2015 (16) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 15 | 14.9 | 20.3 | 100 | Triptorelin 3.75 mg every 4 wk SC from 11.4–18.3 y. | GnRH 1.3 y (range, 0.5–3.8) and CSH therapy 5.8 y (range, 3.0–8.0) |
| In the age range from 15.6 to 19 y transgender women were prescribed incremental dosing of 17-estradiol. | ||||||||||
| At a minimum age of 18 y, after gonadectomy, GnRH treatment was terminated and CSH therapy continued. | ||||||||||
| Same subjects (before and after) | FTM | 19 | 15.0 | 20.9 | 100 | Testosterone esters (Sustanon 250 mg/mL) every 2–4 wk in incremental dosages. | GnRH 1.5 y (range, 0.25–5.2) and CSH 5.4 y (range, 2.8–7.8) | |||
| At a minimum age of 18 y, after gonadectomy, GnRH treatment was terminated and CSH therapy continued. | ||||||||||
| Mueller et al., 2011 (18) | Germany | Cohort | Same subjects (before and after) | MTF | 84 | 36.3 | 22.3 | 0 | 3.8 mg goserelin every 4 wk and a dosage of 10 mg estradiol 17-valerate IM every 10 d. | 24 mo |
| Mueller et al., 2010 (17) | Germany | Cohort | Same subjects (before and after) | FTM | 45 | 30.4 | 24.1 | 0 | Testosterone undecanoate 1000 mg IM every 12 wk. | 12 and 24 mo |
| Pelusi et al., 2014 (19) | Italy | Cohort | Same subjects (before and after) | FTM | 15 | 30.9 | NA | 0 | Testosterone enanthate IM at a dosage of 100 mg every 10 d (n = 15; TD group). | 12 mo |
| Same subjects (before and after) | FTM | 15 | 29.4 | NA | 0 | Testosterone gel at a dosage of 50 mg/d every evening. | 12 mo | |||
| Same subjects (before and after) | FTM | 15 | 28.2 | NA | 0 | Testosterone undecanoate at a dosage of 1000 mg at wk 0, wk 6, and thereafter, every 12 wk. | 12 mo | |||
| Reutrakul et al., 1998 (20) | Thailand | Cohort | Controls | MTF | 11 | 21.2 | NA | 0 | Estradiol valerate 10 mg per ampule, mestranol 0.05 mg norethisterone 1 mg, or contraceptive pills, ethinyl estradiol, and several doses of levonorgestrel. | <24 mo |
| Controls | MTF | 17 | 24.1 | NA | 0 | NA. | >24 mo | |||
| Sosa et al., 2003 (21) | Spain | Cohort | Controls | MTF | 27 | 43 | 26 | 0 | Contraceptive pills (ethinyl estradiol + cyproterone acetate or levonorgestrel), oral estrogen (conjugated equine), and depot estrogens (estradiol valerate or mestranol + norethisterone). | 201 mo (108)b (range 3–35 y) |
| Turner et al., 2004 (22) | USA | Cohort | Same subjects (before and after) | FTM | 8 | 33.1 | NA | 0 | Testosterone IM (mean dosage of 70.4 ±4.5 mg weekly). | 24 mo |
| Van Caenegem et al., 2015 (24) | Belgium | Cohort | Same subjects (before and after) | FTM | 23 | 27 | 24.5 | NA | Testosterone undecanoate of 1000 mg IM. Injections were administered at baseline, after 6 and 18 wk, and from then once every 12 wk. | 12 mo |
| Van Caenegem et al., 2015 (23) | Belgium | Cohort | Same subjects (before and after) | MTF | 49 | 33 | NA | 0 | Oral estradiol valerate, 4 mg daily or transdermal 17-β estradiol 100 μg/24 h for patients >45 years old or both combined with oral cyproterone acetate 50 mg daily. Transdermal estrogens were used in older transgender women because they carry a lower thromboembolic risk. | 12 and 24 mo |
| van Kesteren et al., 1996 (15) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 56 | 33 | 22 | 0 | Cyproterone acetate 100 mg/d with ethinylestradiol or transdermal estradiol twice a week. | 12 mo |
| FTM | 35 | 25 | 23 | 0 | Testosterone esters IM every 2 wk, Sustanon 250, or testosterone undecanoate 160 mg/d orally. | 12 mo | ||||
| van Kesteren et al., 1998 (25) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 20 | 25.4 | 22.1 | 100 | Cyproterone acetate 100 mg/d in combination with ethinylestradiol 100 μg/d until gonadectomy; after surgery just estrogen to maintain female features. | 45.5 mo (9.7)b (range, 32–63) |
| Same subjects (before and after) | FTM | 19 | 25.0 | 22.1 | 100 | Testosterone esters 250 mg/2 wk; after gonadectomy most patients continued with IM every 2–3 wk. | 38.2 mo (8.6)b (range, 28–53) | |||
| Wierckx et al., 2014 (26)c | Belgium | Cohort | Same subjects (before and after)/no comparison | MTF | 47 | 31.7 | 23.9 | NA | 50 mg cyproterone acetate and 4 mg estradiol valerate daily, whereas those >45 y old received 50 mg CA daily together with 100 μg/24 h transdermal 17-β estradiol. | 12 mo |
| Same subjects (before and after)/no comparison | MTF | 6 | 19.3 | 22.9 | NA | 50 mg cyproterone acetate and 4 mg estradiol valerate daily, whereas those >45 y old received 50 mg CA daily together with 100 μg/24 h transdermal 17-β estradiol. | 12 mo | |||
| Same subjects (before and after)/no comparison | FTM | 27 | 27.3 | 24.5 | NA | Testosterone undecanoate IM every 3 mo. | 12 mo | |||
| Same subjects (before and after)/no comparison | FTM | 26 | 21.7 | 25.2 | NA | Testosterone undecanoate IM every 3 mo. | 12 mo |
Abbreviations: BMI, body mass index; CA, cyproterone acetate; CSH, cross-sex hormone; F, female; GCS, gender-confirming surgery; IM, intramuscular; M, male; NA, not available; SC, subcutaneous.
Median.
Mean (standard deviation).
Study reporting fracture data.
Characteristics of Included Studies
| Study . | Country . | Design . | Comparison Group . | Patients . | No. of Patients . | Mean Age . | Mean BMI . | % of Patients with GCS . | Description of Intervention . | Duration of Exposure . |
|---|---|---|---|---|---|---|---|---|---|---|
| Dittrich et al., 2005 (14) | Germany | Cohort | Same subjects (before and after) | MTF | 60 | 38.37 | 24.19a | 0 | 3.8 mg goserelin every 4 wk and 6 mg oral estradiol-17 valerate per d. | 24 mo |
| Klink et al., 2015 (16) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 15 | 14.9 | 20.3 | 100 | Triptorelin 3.75 mg every 4 wk SC from 11.4–18.3 y. | GnRH 1.3 y (range, 0.5–3.8) and CSH therapy 5.8 y (range, 3.0–8.0) |
| In the age range from 15.6 to 19 y transgender women were prescribed incremental dosing of 17-estradiol. | ||||||||||
| At a minimum age of 18 y, after gonadectomy, GnRH treatment was terminated and CSH therapy continued. | ||||||||||
| Same subjects (before and after) | FTM | 19 | 15.0 | 20.9 | 100 | Testosterone esters (Sustanon 250 mg/mL) every 2–4 wk in incremental dosages. | GnRH 1.5 y (range, 0.25–5.2) and CSH 5.4 y (range, 2.8–7.8) | |||
| At a minimum age of 18 y, after gonadectomy, GnRH treatment was terminated and CSH therapy continued. | ||||||||||
| Mueller et al., 2011 (18) | Germany | Cohort | Same subjects (before and after) | MTF | 84 | 36.3 | 22.3 | 0 | 3.8 mg goserelin every 4 wk and a dosage of 10 mg estradiol 17-valerate IM every 10 d. | 24 mo |
| Mueller et al., 2010 (17) | Germany | Cohort | Same subjects (before and after) | FTM | 45 | 30.4 | 24.1 | 0 | Testosterone undecanoate 1000 mg IM every 12 wk. | 12 and 24 mo |
| Pelusi et al., 2014 (19) | Italy | Cohort | Same subjects (before and after) | FTM | 15 | 30.9 | NA | 0 | Testosterone enanthate IM at a dosage of 100 mg every 10 d (n = 15; TD group). | 12 mo |
| Same subjects (before and after) | FTM | 15 | 29.4 | NA | 0 | Testosterone gel at a dosage of 50 mg/d every evening. | 12 mo | |||
| Same subjects (before and after) | FTM | 15 | 28.2 | NA | 0 | Testosterone undecanoate at a dosage of 1000 mg at wk 0, wk 6, and thereafter, every 12 wk. | 12 mo | |||
| Reutrakul et al., 1998 (20) | Thailand | Cohort | Controls | MTF | 11 | 21.2 | NA | 0 | Estradiol valerate 10 mg per ampule, mestranol 0.05 mg norethisterone 1 mg, or contraceptive pills, ethinyl estradiol, and several doses of levonorgestrel. | <24 mo |
| Controls | MTF | 17 | 24.1 | NA | 0 | NA. | >24 mo | |||
| Sosa et al., 2003 (21) | Spain | Cohort | Controls | MTF | 27 | 43 | 26 | 0 | Contraceptive pills (ethinyl estradiol + cyproterone acetate or levonorgestrel), oral estrogen (conjugated equine), and depot estrogens (estradiol valerate or mestranol + norethisterone). | 201 mo (108)b (range 3–35 y) |
| Turner et al., 2004 (22) | USA | Cohort | Same subjects (before and after) | FTM | 8 | 33.1 | NA | 0 | Testosterone IM (mean dosage of 70.4 ±4.5 mg weekly). | 24 mo |
| Van Caenegem et al., 2015 (24) | Belgium | Cohort | Same subjects (before and after) | FTM | 23 | 27 | 24.5 | NA | Testosterone undecanoate of 1000 mg IM. Injections were administered at baseline, after 6 and 18 wk, and from then once every 12 wk. | 12 mo |
| Van Caenegem et al., 2015 (23) | Belgium | Cohort | Same subjects (before and after) | MTF | 49 | 33 | NA | 0 | Oral estradiol valerate, 4 mg daily or transdermal 17-β estradiol 100 μg/24 h for patients >45 years old or both combined with oral cyproterone acetate 50 mg daily. Transdermal estrogens were used in older transgender women because they carry a lower thromboembolic risk. | 12 and 24 mo |
| van Kesteren et al., 1996 (15) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 56 | 33 | 22 | 0 | Cyproterone acetate 100 mg/d with ethinylestradiol or transdermal estradiol twice a week. | 12 mo |
| FTM | 35 | 25 | 23 | 0 | Testosterone esters IM every 2 wk, Sustanon 250, or testosterone undecanoate 160 mg/d orally. | 12 mo | ||||
| van Kesteren et al., 1998 (25) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 20 | 25.4 | 22.1 | 100 | Cyproterone acetate 100 mg/d in combination with ethinylestradiol 100 μg/d until gonadectomy; after surgery just estrogen to maintain female features. | 45.5 mo (9.7)b (range, 32–63) |
| Same subjects (before and after) | FTM | 19 | 25.0 | 22.1 | 100 | Testosterone esters 250 mg/2 wk; after gonadectomy most patients continued with IM every 2–3 wk. | 38.2 mo (8.6)b (range, 28–53) | |||
| Wierckx et al., 2014 (26)c | Belgium | Cohort | Same subjects (before and after)/no comparison | MTF | 47 | 31.7 | 23.9 | NA | 50 mg cyproterone acetate and 4 mg estradiol valerate daily, whereas those >45 y old received 50 mg CA daily together with 100 μg/24 h transdermal 17-β estradiol. | 12 mo |
| Same subjects (before and after)/no comparison | MTF | 6 | 19.3 | 22.9 | NA | 50 mg cyproterone acetate and 4 mg estradiol valerate daily, whereas those >45 y old received 50 mg CA daily together with 100 μg/24 h transdermal 17-β estradiol. | 12 mo | |||
| Same subjects (before and after)/no comparison | FTM | 27 | 27.3 | 24.5 | NA | Testosterone undecanoate IM every 3 mo. | 12 mo | |||
| Same subjects (before and after)/no comparison | FTM | 26 | 21.7 | 25.2 | NA | Testosterone undecanoate IM every 3 mo. | 12 mo |
| Study . | Country . | Design . | Comparison Group . | Patients . | No. of Patients . | Mean Age . | Mean BMI . | % of Patients with GCS . | Description of Intervention . | Duration of Exposure . |
|---|---|---|---|---|---|---|---|---|---|---|
| Dittrich et al., 2005 (14) | Germany | Cohort | Same subjects (before and after) | MTF | 60 | 38.37 | 24.19a | 0 | 3.8 mg goserelin every 4 wk and 6 mg oral estradiol-17 valerate per d. | 24 mo |
| Klink et al., 2015 (16) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 15 | 14.9 | 20.3 | 100 | Triptorelin 3.75 mg every 4 wk SC from 11.4–18.3 y. | GnRH 1.3 y (range, 0.5–3.8) and CSH therapy 5.8 y (range, 3.0–8.0) |
| In the age range from 15.6 to 19 y transgender women were prescribed incremental dosing of 17-estradiol. | ||||||||||
| At a minimum age of 18 y, after gonadectomy, GnRH treatment was terminated and CSH therapy continued. | ||||||||||
| Same subjects (before and after) | FTM | 19 | 15.0 | 20.9 | 100 | Testosterone esters (Sustanon 250 mg/mL) every 2–4 wk in incremental dosages. | GnRH 1.5 y (range, 0.25–5.2) and CSH 5.4 y (range, 2.8–7.8) | |||
| At a minimum age of 18 y, after gonadectomy, GnRH treatment was terminated and CSH therapy continued. | ||||||||||
| Mueller et al., 2011 (18) | Germany | Cohort | Same subjects (before and after) | MTF | 84 | 36.3 | 22.3 | 0 | 3.8 mg goserelin every 4 wk and a dosage of 10 mg estradiol 17-valerate IM every 10 d. | 24 mo |
| Mueller et al., 2010 (17) | Germany | Cohort | Same subjects (before and after) | FTM | 45 | 30.4 | 24.1 | 0 | Testosterone undecanoate 1000 mg IM every 12 wk. | 12 and 24 mo |
| Pelusi et al., 2014 (19) | Italy | Cohort | Same subjects (before and after) | FTM | 15 | 30.9 | NA | 0 | Testosterone enanthate IM at a dosage of 100 mg every 10 d (n = 15; TD group). | 12 mo |
| Same subjects (before and after) | FTM | 15 | 29.4 | NA | 0 | Testosterone gel at a dosage of 50 mg/d every evening. | 12 mo | |||
| Same subjects (before and after) | FTM | 15 | 28.2 | NA | 0 | Testosterone undecanoate at a dosage of 1000 mg at wk 0, wk 6, and thereafter, every 12 wk. | 12 mo | |||
| Reutrakul et al., 1998 (20) | Thailand | Cohort | Controls | MTF | 11 | 21.2 | NA | 0 | Estradiol valerate 10 mg per ampule, mestranol 0.05 mg norethisterone 1 mg, or contraceptive pills, ethinyl estradiol, and several doses of levonorgestrel. | <24 mo |
| Controls | MTF | 17 | 24.1 | NA | 0 | NA. | >24 mo | |||
| Sosa et al., 2003 (21) | Spain | Cohort | Controls | MTF | 27 | 43 | 26 | 0 | Contraceptive pills (ethinyl estradiol + cyproterone acetate or levonorgestrel), oral estrogen (conjugated equine), and depot estrogens (estradiol valerate or mestranol + norethisterone). | 201 mo (108)b (range 3–35 y) |
| Turner et al., 2004 (22) | USA | Cohort | Same subjects (before and after) | FTM | 8 | 33.1 | NA | 0 | Testosterone IM (mean dosage of 70.4 ±4.5 mg weekly). | 24 mo |
| Van Caenegem et al., 2015 (24) | Belgium | Cohort | Same subjects (before and after) | FTM | 23 | 27 | 24.5 | NA | Testosterone undecanoate of 1000 mg IM. Injections were administered at baseline, after 6 and 18 wk, and from then once every 12 wk. | 12 mo |
| Van Caenegem et al., 2015 (23) | Belgium | Cohort | Same subjects (before and after) | MTF | 49 | 33 | NA | 0 | Oral estradiol valerate, 4 mg daily or transdermal 17-β estradiol 100 μg/24 h for patients >45 years old or both combined with oral cyproterone acetate 50 mg daily. Transdermal estrogens were used in older transgender women because they carry a lower thromboembolic risk. | 12 and 24 mo |
| van Kesteren et al., 1996 (15) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 56 | 33 | 22 | 0 | Cyproterone acetate 100 mg/d with ethinylestradiol or transdermal estradiol twice a week. | 12 mo |
| FTM | 35 | 25 | 23 | 0 | Testosterone esters IM every 2 wk, Sustanon 250, or testosterone undecanoate 160 mg/d orally. | 12 mo | ||||
| van Kesteren et al., 1998 (25) | The Netherlands | Cohort | Same subjects (before and after) | MTF | 20 | 25.4 | 22.1 | 100 | Cyproterone acetate 100 mg/d in combination with ethinylestradiol 100 μg/d until gonadectomy; after surgery just estrogen to maintain female features. | 45.5 mo (9.7)b (range, 32–63) |
| Same subjects (before and after) | FTM | 19 | 25.0 | 22.1 | 100 | Testosterone esters 250 mg/2 wk; after gonadectomy most patients continued with IM every 2–3 wk. | 38.2 mo (8.6)b (range, 28–53) | |||
| Wierckx et al., 2014 (26)c | Belgium | Cohort | Same subjects (before and after)/no comparison | MTF | 47 | 31.7 | 23.9 | NA | 50 mg cyproterone acetate and 4 mg estradiol valerate daily, whereas those >45 y old received 50 mg CA daily together with 100 μg/24 h transdermal 17-β estradiol. | 12 mo |
| Same subjects (before and after)/no comparison | MTF | 6 | 19.3 | 22.9 | NA | 50 mg cyproterone acetate and 4 mg estradiol valerate daily, whereas those >45 y old received 50 mg CA daily together with 100 μg/24 h transdermal 17-β estradiol. | 12 mo | |||
| Same subjects (before and after)/no comparison | FTM | 27 | 27.3 | 24.5 | NA | Testosterone undecanoate IM every 3 mo. | 12 mo | |||
| Same subjects (before and after)/no comparison | FTM | 26 | 21.7 | 25.2 | NA | Testosterone undecanoate IM every 3 mo. | 12 mo |
Abbreviations: BMI, body mass index; CA, cyproterone acetate; CSH, cross-sex hormone; F, female; GCS, gender-confirming surgery; IM, intramuscular; M, male; NA, not available; SC, subcutaneous.
Median.
Mean (standard deviation).
Study reporting fracture data.
Risk of bias
We judged the observational studies to be at moderate risk of bias. The cohorts selected in most studies appeared to represent the totality of practice experience (as opposed to selected cases), and outcomes were ascertained via secured medical record review (Supplemental Table 1).
Meta-analysis
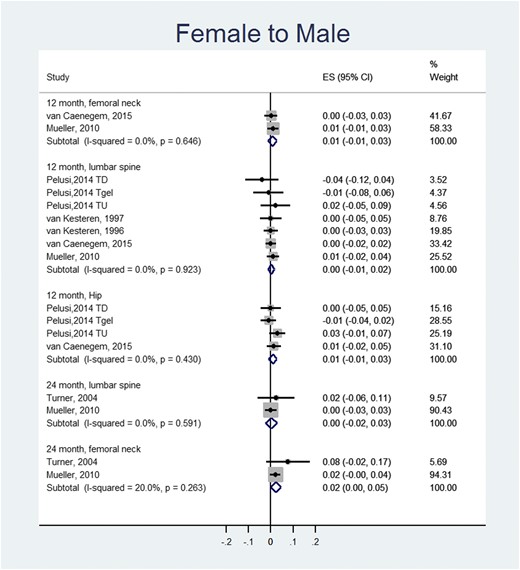
In FTM transgender individuals, meta-analysis showed no statistically significant changes in the lumbar spine, femoral neck, and total hip BMD at 12 and 24 months after initiation of treatment compared with baseline values (Fig. 1).
Meta-analysis of BMD changes (g/cm2) in FTM individuals (compared to baseline values). ES, effect size; a positive value suggests increase in BMD after receiving hormone therapy. TD, testosterone depot; TU, testosterone undeconoate.
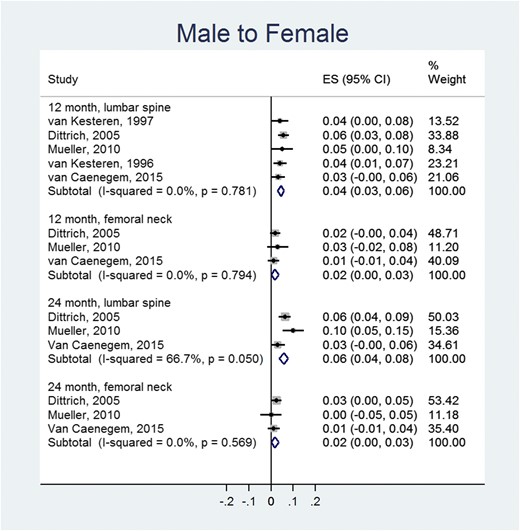
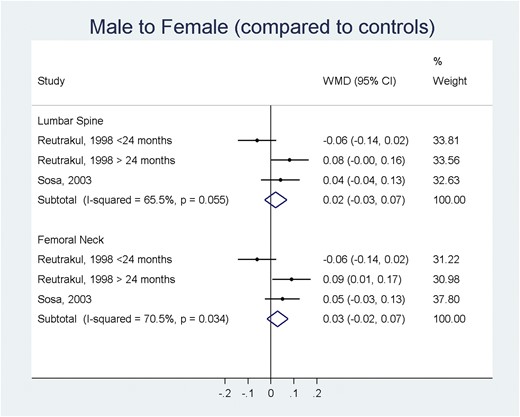
In the MTF group and compared with baseline values, there was a statistically significant increase of BMD at 12 (0.04 g/cm2; 95% CI, 0.03 to 0.06 g/cm2) and 24 months at the lumbar spine (0.06 g/cm2; 95% CI, 0.04 to 0.08 g/cm2) (Fig. 2). Changes in femoral neck BMD were not statistically significant. The changes in BMD when the transgender MTF group was compared with a control group (genetic sex) were not statistically significant at the lumbar spine or the femoral neck (Fig. 3).
Meta-analysis of BMD changes (g/cm2) in MTF individuals (compared to baseline values). ES, effect size; a positive value suggests increase in BMD after receiving hormone therapy.
Meta-analysis of BMD changes (g/cm2) in MTF individuals (compared to a control group). ES, effect size; a positive value suggests increase in BMD compared with a control group of a matching genetic sex.
One study assessed fracture rates during follow-up. In this study, 53 MTF and 53 FTM patients had no events (fractures) for 12 months of follow-up (26). A single study evaluated the effect of GnRH administration (median of 1.3 years for the MTF group, 1.5 years for the FTM group) and sex steroid therapy on adolescents and found the BMD at the lumbar spine to be lower in MTF at 22 years of age when compared with baseline and a trend to lower BMD values when compared with baseline in the FTM group (16).
Subgroup and sensitivity analysis
We performed a sensitivity analysis of the change of BMD in FTM individuals for studies where testosterone was administered only IM, without any changes in the results compared with the main analysis (Supplemental Table 2). Because of inconsistent reporting, we were unable to conduct the other preplanned subgroup analyses.
Discussion
We performed a systematic review and meta-analysis to summarize the effect of sex steroid therapy on the bone health of transgender individuals. The effects of this therapy on the rate of fractures were evaluated only in a small study (53 MTF, 53 FTM) of short duration (12 months of follow-up), with no fractures reported in either of the two groups. Similarly, the effects of GnRH agonists and sex steroids on the bone health of adolescents were evaluated in only one study (16). The majority of the available studies evaluated changes in BMD in young individuals undergoing sex steroid therapy. In the FTM individuals who received masculinizing therapy, no statistically significant changes on BMD were found when compared with baseline at the femoral neck, lumbar spine, or total hip at 12 months or in the lumbar spine or femoral neck after 24 months of therapy. These results continued to be statistically nonsignificant when only studies including patients who received IM testosterone were analyzed. On the other hand, in MTF individuals who received feminizing therapy there was an increase in BMD at the lumbar spine at both 12 and 24 months after therapy; no statistically significant changes were noted in the femoral neck or total hip. The body of evidence is derived from small observational studies of short follow-up time at moderate risk of bias.
Strengths and limitations
We conducted an extensive search of the available literature without any language restriction with the help of a medical librarian, which decreases the chances of missing studies addressing this clinical question (31). In addition, we performed each step of the review in duplicate with good agreement. We attempted to decrease the chances of reporting bias by contacting authors; however, the response rate was low (32). The results of the review were overall consistent across studies, yet we were unable to perform most of our preplanned subgroup analysis because the outcomes were not consistently reported across different subgroups.
Implications for practice
Clinicians and transgender individuals benefit from information that can clarify the benefits (e.g., overall quality of life, psychological distress) and potential harms of sex steroid therapy (e.g., mortality, cancer risk, bone health). Our findings suggest that in FTM individuals, masculinizing therapy is not associated with significant changes in BMD 1 and 2 years after therapy is initiated. On the other hand, in MTF individuals, feminizing therapy may be associated with improvement of BMD at the lumbar spine. However, because BMD is a surrogate marker for overall bone health, the effect of these findings on patient-important outcomes such as fractures during long-term therapy remains unknown. In addition, it is a challenge to determine the clinical impact and relevance of BMD changes (because of precision issues and variation in measurements and its overall effect on fracture rates) (33, 34). For example, BMD changes of 0.022 g/cm2 per year at the spine and 0.013 g/cm2 at the hip have been reported in postmenopausal women and are considered significant (35). In our analysis, the magnitude of BMD change in the MTF group at the lumbar spine was found to be in that range.
Implications for research
We have identified many clinical knowledge gaps regarding the effects of sex steroid therapy on bone health. First, there is paucity of data regarding fracture rates in this population, and the studies evaluating BMD changes have had a small number of individuals enrolled and short follow-up times. Second, literature addressing this clinical question in the pediatric and adolescent population is lacking, with a single study evaluating the effect of sex steroid treatment in this population. In addition, the mean age of the individuals in the included adult studies ranged from 19 to 43 years, suggesting that cross-sex hormone therapy was started in many of these patients in a window where their BMD could have been increasing. Conducting research at low risk of bias in this population can be challenging given the overall barriers to care that these individuals face. However, it is possible that the number of individuals seeking sex steroid therapy in the future to alleviate their psychological burden will continue to increase. Therefore, medical centers that provide care to these individuals should make it a priority to conduct studies evaluating patient-important outcomes during their long-term follow-up, which will benefit both patients and their clinicians (36).
Conclusion
Evidence from small observational studies with short-term follow-up suggests that in FTM individuals sex steroid therapy does not seem to be associated with significant changes in BMD at 12 and 24 months after initiation of therapy. In MTF individuals, sex steroid therapy appears to be associated with increased BMD at the lumbar spine at 12 and 24 months after initiation of therapy. The impact of these BMD changes on patient-important outcomes such as fracture risk remains uncertain.
Abbreviations
- BMD
bone mineral density
- CI
confidence interval
- FTM
female-to-male
- GnRH
gonadotropin-releasing hormone
- IM
intramuscular
- MTF
male-to-female.
Acknowledgments
We thank Drs. Jones and Schagen for responding to our inquiries in regard to their publications.
Financial Support: This systematic review was funded by the Endocrine Society.
Disclosure Summary: The authors have nothing to disclose.
References
Wells GSB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013 Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 2015.
Author notes
Both authors contributed equally to this study.