-
PDF
- Split View
-
Views
-
Cite
Cite
Michelle J. Khan, Philip E. Castle, Attila T. Lorincz, Sholom Wacholder, Mark Sherman, David R. Scott, Brenda B. Rush, Andrew G. Glass, Mark Schiffman, The Elevated 10-Year Risk of Cervical Precancer and Cancer in Women With Human Papillomavirus (HPV) Type 16 or 18 and the Possible Utility of Type-Specific HPV Testing in Clinical Practice, JNCI: Journal of the National Cancer Institute, Volume 97, Issue 14, 20 July 2005, Pages 1072–1079, https://doi.org/10.1093/jnci/dji187
Close - Share Icon Share
Abstract
Background: Human papillomavirus (HPV) types 16 and 18 cause 60%–70% of cervical cancer worldwide, and other HPV types cause virtually all remaining cases. Pooled HPV testing for 13 oncogenic types, including HPV16 and 18, is currently used in clinical practice for triage of equivocal cytology and, in conjunction with Pap tests, is an option for general screening among women 30 years of age and older. It is not clear to what extent individual identification of HPV16 or HPV18 as an adjunct to pooled oncogenic HPV testing might effectively identify women at particularly high risk of cervical cancer or its immediate precursor, cervical intraepithelial neoplasia 3 (CIN3). Methods: From April 1, 1989, to November 2, 1990, a total of 20 810 women in the Kaiser Permanente health plan in Portland, OR, enrolled in a cohort study of HPV and cervical neoplasia. Women were tested for 13 oncogenic HPV types by Hybrid Capture 2 (HC2), and those women with a positive HC2 test were tested for HPV16 and 18. Enrollment Pap smear interpretation and HPV test results were linked to histologically confirmed CIN3 and cervical cancer (≥CIN3) occurring during 10 years of cytologic follow-up. We calculated cumulative incidence rates with 95% confidence intervals for each interval up to 122 months using Kaplan–Meier methods. Results: The 10-year cumulative incidence rates of ≥CIN3 were 17.2% (95% confidence interval [CI] = 11.5% to 22.9%) among HPV16+ women and 13.6% (95% CI = 3.6% to 23.7%) among HPV18+ (HPV16−) women, but only 3.0% (95% CI = 1.9% to 4.2%) among HC2+ women negative for HPV16 or HPV18. The 10-year cumulative incidence among HC2− women was 0.8% (95% CI = 0.6% to 1.1%). A subanalysis among women 30 years of age and older with normal cytology at enrollment strengthened the observed risk differences. Conclusions: HPV screening that distinguishes HPV16 and HPV18 from other oncogenic HPV types may identify women at the greatest risk of ≥CIN3 and may permit less aggressive management of other women with oncogenic HPV infections.
Infection with human papillomavirus (HPV) causes 95%–100% of all cervical cancer, which is the second most common cancer in women worldwide ( 1 – 3 ) . Of about 40 known sexually transmitted HPV types, approximately 15 have been established as oncogenic (high-risk) types in epidemiologic studies ( 4 – 6 ) . International case–control studies have demonstrated the approximate proportion of squamous cell cervical carcinoma for which each oncogenic HPV type is responsible: HPV16 causes more than 50% of cancers, HPV18 causes 10%–15%, HPV45 causes approximately 7%, and HPV31 causes approximately 3% ( 7 , 8 ) . Other oncogenic HPV types individually cause less than 2% of cervical squamous cell cancer ( 5 ) . HPV18 also causes more than 35% of cervical adenocarcinomas, which are difficult to detect by current cytologic screening methods ( 8 ) . HPV16 and 18 are two of the most common HPV types in women without cancer as well ( 9 ) .
The risk of cervical neoplasia associated with infection by individual HPV types has been examined in cross-sectional and case–control studies, but few studies have examined the prospective risks associated with individual HPV types in the general population. In a prospective cohort of 1075 women 15–19 years old, Woodman et al. ( 10 ) demonstrated that, compared with HPV-negative women, women infected with HPV16 and 18 have relative hazard ratios of 8.5% (95% confidence interval [CI] = 3.7 to 19.2) and 3.3% (95% CI = 1.4 to 8.1), respectively, for development of cervical intraepithelial neoplasia 2 (CIN2) or 3 (CIN3, equivalent to precancer) over a 3-year period after primary infection. In another prospective study of 603 female university students, Winer et al. ( 11 ) reported a cumulative incidence rate for high-grade CIN (CIN2 and CIN3) of 27.2% (95% CI = 16.3 to 43.3) after incident infection with HPV16 or 18. In the natural history of HPV, most infections are transient, especially among younger women; only the small fraction of infections that persist may progress to cervical cancer, usually after more than a decade. Therefore, HPV DNA testing for use in primary screening as an adjunct to cytology has only been approved by the Food and Drug Administration and recommended for women 30 years of age and older ( 12 – 14 ) . However, published prospective data regarding type-specific risks in this age group are still lacking.
The only HPV DNA test currently approved in the United States for co-screening with cytology, Hybrid Capture 2 (HC2), uses a pooled probe set for 13 oncogenic HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68); the test does not distinguish individual HPV types. We recently examined the performance of this test in more than 20 000 women enrolled in a 10-year prospective cohort and found that HC2 demonstrated superior sensitivity and negative predictive value over 5–10 years compared with a single Pap smear ( 15 ) . However, we wondered whether the value of HPV testing could be further optimized by separate detection of the most important HPV types. Specifically, we used type-specific probes for HPV16 and 18 in this same cohort study, to clarify whether additional testing of oncogenic HPV-positive (HC2+) women for HPV16 and HPV18 could better predict the future development of cervical precancer (CIN3) and cancer. If so, the risks associated with these two HPV types might justify serious consideration of HPV16 and HPV18 type-specific testing as an adjunct to a pooled oncogenic HPV DNA test.
S UBJECTS AND M ETHODS
Study Participants
From April 1, 1989, to November 2, 1990, 23 702 women receiving routine cytologic screening in a prepaid health plan at Kaiser Permanente in Portland, OR, were recruited for a cohort study of the natural history of HPV infection. Women were excluded as described previously ( 15 , 16 ) , and the remaining cohort of 20 810 women with satisfactory baseline cytology was followed prospectively by routine cytology for up to 122 months. The cohort was a demographically representative sample (mainly Caucasian) in which approximately 50% of women underwent cervical cytologic screening at Kaiser Permanente, which served about one-quarter of the women residing in Portland during this time.
After exclusion of 208 women with indeterminate baseline cytology, 51 women with high-grade squamous intraepithelial lesions (HSILs) or cancer cytology at baseline, and 37 women who tested positive for oncogenic HPV types but did not have HPV16 or HPV18 typing results, the current analysis was restricted to 20 514 women with negative, equivocal, or mildly abnormal baseline cervical Pap smears; suitable samples for HPV testing; and applicable type-specific HPV test results. Subjects were 16 years of age or older (median age = 34.0 years, standard deviation [SD] = 12.6 years). Separate analyses were performed on the subgroup of 13 229 women aged 30 years or older at enrollment to address current age–specific screening recommendations ( 12 , 13 ) .
Enrollment Examination
Informed consent was obtained under the prevailing institutional review board guidelines at Kaiser Permanente and the National Institutes of Health. Participants underwent a routine pelvic examination. Experienced clinicians prepared a single ethanol-fixed Pap smear for each subject using an Ayre spatula and cytobrush. Next, the cervix was rinsed with 10 mL of sterile saline using a 3¼ inch flexible intracatheter extender. The pooled fluid was collected from the posterior vaginal fornix and processed for HPV testing as described below.
Follow-Up
During the study period, annual cytologic screening of women at Kaiser continued as part of standard clinical practice. The then-current standard practice guidelines for management of abnormal cytology mandated treatment of patients with CIN2 or greater, but health plan physicians also treated some patients with CIN1 at their discretion (which is more aggressive treatment than current guidelines recommend) ( 12 , 14 ) . Once treated, women were censored and were not included in the denominator of women at risk in subsequent time intervals. HPV test results were not known by clinicians and were not used to direct patient management.
Pathology
Pap smears were originally reported using a classification that predated the development of the Bethesda System; we converted these interpretations into Bethesda 2001 terminology for this study ( 17 ) . We reclassified women with smears reported as “normal” or “benign reactive atypia” as “negative for intraepithelial lesion or malignancy (negative)” according to the Bethesda 2001 classification ( 17 ) . Pap smears reported as “severe reactive atypia, possibly dysplasia” or “possible koilocytotic or condylomatous atypia” were reclassified as “atypical squamous cells” (ASCs). Cytologic interpretations of dysplasia were reclassified as low-grade squamous intraepithelial lesions (LSILs) or HSILs. Histologic diagnoses were converted into CIN nomenclature. Specifically, severe dysplasia and carcinoma in situ were categorized as CIN3.
Women who had received original histopathologic diagnoses of CIN3 or cancer (including endocervical adenocarcinoma in situ) on two different clinical specimens obtained on different dates (usually a diagnostic punch biopsy and a cone performed for treatment) were designated as cases, called ≥CIN3, and were not further reviewed. All other women who had a CIN2 or greater histopathology result underwent histologic specimen review. A single pathologist (DRS) performed the reviews. The review criteria for case definition were 1) an original histopathologic diagnosis of CIN2 reviewed as CIN3 or worse or 2) an original histopathologic diagnosis of CIN3 or worse confirmed as at least CIN2. This case definition, which required confirmation of a single CIN3 diagnosis as at least CIN2 by another pathologist, was more stringent than a disease endpoint defined by a single pathologist. For example, an original diagnosis of CIN3 that was reviewed as CIN1 would not have been a case in our analysis. We chose these criteria because we wished, by review, to exclude questionable precancer; however, the subtle histopathologic distinction between CIN2 and CIN3 has inadequate reproducibility, even among experts ( 18 ) . Therefore, in total, 131 (0.6%) of 20 514 women fulfilled this ≥CIN3 case definition, including 32 (0.2%) subjects with invasive carcinoma.
HPV DNA Testing
Cervicovaginal lavage specimens were refrigerated within 1 hour of collection and transported to a laboratory for processing. A 1-mL aliquot was removed and frozen at −70°C ( 19 ) . The remaining sample was divided roughly in half, cells were pelleted by centrifugation, the supernatant was separated from the pellet, and both were frozen.
We selected either frozen liquid aliquots or cell pellets for HPV testing, depending on availability. The vast majority of specimens were tested using cell pellets (92%). Separate analysis of the few specimens tested using liquid aliquots (8%) did not change our conclusions (data not shown). HPV testing (by laboratory personnel who were blinded to cytology and clinical outcome) was performed on enrollment specimens using the HC2 microplate assay at a detection threshold of 1.0 pg/mL (approximately 5000 copies). The assay detected 13 oncogenic types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), as previously described (Digene, Gaithersburg, MD) ( 20 , 21 ) . As a method of secondary typing, we performed HPV16 and HPV18 testing using individual type-specific RNA probes coupled with type-specific capture of DNA:RNA hybrids using immobilized DNA oligonucleotides, as described previously ( 22 , 23 ) , on women who were HC2 positive ( n = 2853). The Hybrid Capture (HC) genotyping method previously had been called the HC3 test and is described briefly as follows. Clinical specimens were denatured by heating in alkali to separate all DNA strands, as described previously for the HC2 test ( 21 ) . Then one almost-full–genome-length unlabeled RNA probe with short deletions in regions that correspond to two separate points of capture at least 3 kb apart on the genome of each target HPV type was combined with two small DNA capture oligonucleotides for each HPV type. The capture oligonucleotides exactly matched each target, and these were labeled with biotin. The purpose of the deletions in the RNA probes was to allow free access of the capture oligonucleotides to any HPV DNA targets that may have been present in the clinical specimens. These two kinds of probes, along with two pairs of short corresponding blocking oligonucleotides designed to suppress any residual cross-reactivity, were allowed to hybridize to target HPV DNA. The capture and corresponding blocking oligonucleotide pairs were chosen to hybridize only to specific unique regions of the HPV target to minimize or eliminate unwanted cross-reactivity. These multipart hybrid complexes were then captured on streptavidin-coated plates, washed to remove unreacted molecules, and detected by supplying a dioxetane substrate as in the HC2 test.
To examine the sensitivity of the initial HC2 testing for detection of HPV16 and HPV18 infections, we analyzed additional available type-specific results using HPV16 and HPV18 RNA probes in a nonrandomly chosen group of women who were HC2 negative ( n = 1381). Many of these women had some other evidence of cervical cancer risk factors or HPV infection using other testing methods ( 23 ) , and we used their HPV16 and HPV18 type-specific results as well as their final diagnosis to assess the analytic and clinical sensitivity of the initial HC2 test for oncogenic HPV types and clinically relevant infection.
Statistical Analysis
First, we divided the entire analysis cohort of 20 514 women into risk-stratified groups based on their HPV status at enrollment. Using HC2 results and HPV16 and HPV18 type-specific probe results, HPV infection was defined hierarchically: positive for HPV16 (HPV16+); else positive for HPV18 (HPV18+; 30 women with HPV16 coinfection were called HPV16+); else HPV16 negative, HPV18 negative, and HC2 positive (HPV16−/HPV18−/HC2+); else HC2 negative (HC2−). Of the 20 514 women, we classified 460 (2.2%) as HPV16+, 157 (0.8%) as HPV18+, 2,236 (10.9%) as HPV16−/HPV18-/HC2+, and 17 661 (86.1%) as HC2−.
Enrollment Pap smears were grouped by cytology: negative, ASCs, and LSILs. Of the 20 514 women, 19 919 (97.1%) had negative cytology at enrollment, 471 (2.3%) had ASCs, and 124 (0.6%) had LSILs.
We purposely de-emphasized exact time of diagnosis of ≥CIN3, because our experience strongly indicates that even repeated screening or expert colposcopic evaluation may miss many cases that, when detected at a later time, may be substantially misclassified as to time of development ( 24 , 25 ) . Therefore, after excluding women who had cytologic evidence of CIN2–3 or cancer at baseline, we included all subsequent cases of histologically confirmed ≥CIN3 through 122 months to examine the cumulative risk for ≥CIN3 over a 10-year period without attempting to assign exact date of occurrence. Instead, follow-up time was crudely divided into an initial period of 0–9 months (Pap smears that were rapidly repeated, presumably prompted by a previous cytologic abnormality or suspicious symptoms), followed by yearly intervals for a total time of 122 months. These intervals roughly paralleled the intervals at which women returned for annual smears.
The risk of ≥CIN3 in each of the four HPV groups (HPV16+, HPV18+, HPV16−/HPV18−/HC2+, and HC2−) for each time interval was computed by dividing the number of cases diagnosed in that interval by the number of women at risk (i.e., who had undergone routine cytology screening) during that interval. Using Kaplan–Meier methods ( 26 ) , we calculated cumulative incidence rates (CIRs) with 95% confidence intervals for each interval up to 122 months. The CIR among women with positive screening tests is the positive predictive value (i.e., number of cases of ≥CIN3 among women with positive tests, divided by total number of positive tests, multiplied by 100%), adjusted for person-time and censoring. Similarly, the negative predictive value, adjusted for person-time and censoring, is equal to 100% minus the CIR in women with negative screening tests. Graphs were plotted to show the trend in CIR over the 10-year period.
We repeated the analysis after stratifying by age (<30 years versus ≥30 years) to evaluate the clinical application of HPV genotyping among older women for whom HPV and cytology co-testing is an option ( 12 – 14 ) . To the extent possible, given the limited numbers of women in each group, we also considered possible modifications of results by enrollment Pap smear result (negative, ASCs, or LSILs).
R ESULTS
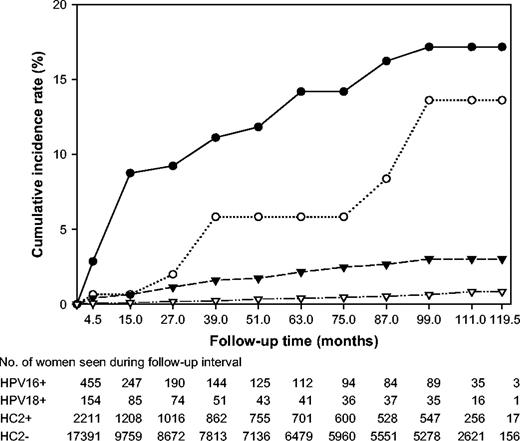
The overall CIRs of ≥CIN3 in 20 514 women according to HPV status at enrollment are shown in Fig. 1 . Over a period of 10 years, 39 women who were HPV16+ at enrollment developed CIN3 or cancer (CIR = 17.2%, 95% CI = 11.5% to 22.9%), as did seven HPV18+ women (CIR = 13.6%, 95% CI = 3.6% to 23.7%), 30 HPV16−/HPV18−/HC2+ women (CIR = 3.0%, 95% CI = 1.9% to 4.2%), and 55 HC2−women (CIR = 0.8%, 95% CI = 0.6% to 1.1%). HPV16+ and HPV18+ women were at increased risk for ≥CIN3 in each time interval up to 8 years after enrollment. Of the 32 women who developed cancer, 12 (37.5%) were HPV16+ at enrollment, one (3.1%) was HPV18+, eight (25.0%) were HPV16−/HPV18−/HC2+, and 11 (34.4%) were HC2−. Of the 99 women who developed CIN3, 27 (27.3%) were HPV16+, 6 (6.1%) were HPV18+, 22 (22.2%) were HPV16−/HPV18−/HC2+, and 44 (44.4%) were HC2− at enrollment. An examination of the absolute risk of ≥CIN3 in each follow-up interval by HPV status also demonstrated that HPV16 and 18 were associated with higher risks than non-HPV16/18 oncogenic types and oncogenic HPV negativity (Supplementary Table 1, available at http://jncicancerspectrum.oxfordjournals.org/jnci/content/vol97/issue14 ).
Cumulative incidence of cervical intraepithelial neoplasia grade 3 and cancer (≥CIN3) over a 10-year period in 20 514 women according to oncogenic human papillomavirus (HPV) status at enrollment. HPV status is defined hierarchically as: positive for HPV 16 ( closed circles ), else positive for HPV18 ( open circles ), else positive for the non-HPV16/18 oncogenic types in Hybrid Capture 2 ( closed triangles ), else oncogenic HPV negative ( open triangles ).
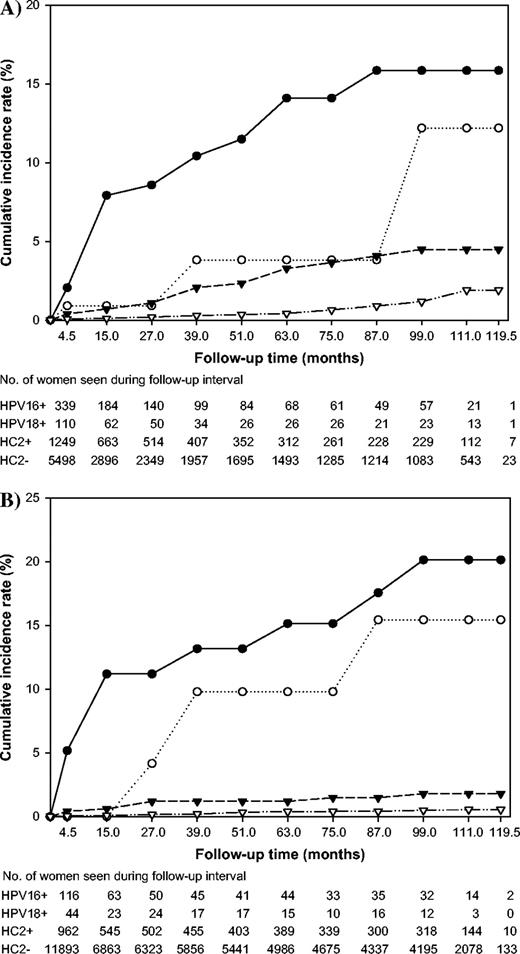
We then stratified the analysis by age and enrollment cytology to examine the risks in subgroups of women who might be targeted for different clinical management strategies. The CIRs for the 7285 women younger than 30 years of age and the 13 229 women 30 years of age and older are shown ( Fig. 2, A and B , respectively). HPV DNA co-screening with cytology is now an option for some women aged 30 years or more (i.e., the women in Fig. 2, B ). The overall rate of ≥CIN3 was 0.4% in the women aged 30 years and older and 1.0% in the women younger than 30 years of age (data not shown). The mean ages of women with CIN3 and cancer were 29.7 (SD = 9.4; range = 16–62) years and 36.8 (SD = 14.2; range = 19–78) years, respectively. Of the 32 women who developed cancer, nine were younger than 30 years of age at baseline and 23 were 30 years of age or older. After stratifying by age, the risks of ≥CIN3 for HPV16+ and HPV18+ women were still substantially elevated above those of HPV16−/HPV18−/HC2+ and HC2− women. However, non-HPV16/18 oncogenic types appeared to contribute more to the development of CIN3 and cancer in younger women ( n = 20 of 73 total cases, CIR = 4.5%, 95% CI = 2.3% to 6.6%) than in older women ( n = 10 of 58 total cases, CIR = 1.8%, 95% CI = 0.6% to 3.0%).
Cumulative incidence of cervical intraepithelial neoplasia grade 3 and cancer (≥CIN3) over a 10-year period in A ) 7285 women younger than 30 years of age and B ) 13 229 women 30 years old and older, according to oncogenic human papillomavirus (HPV) status at enrollment. HPV status is defined hierarchically as: positive for HPV 16 ( closed circles ), else positive for HPV18 ( open circles ), else positive for the non-HPV16/18 oncogenic types in Hybrid Capture 2 (HC2) ( closed triangles ), else oncogenic HPV negative ( open triangles ).
When we excluded women with ASC or LSIL cytology, we found that the risks of ≥CIN3 for 19 919 women who were cytologically negative at enrollment were similar to those for the entire cohort; the risks of ≥CIN3 in HPV16+ ( n = 25, CIR = 17.3%, 95% CI = 10.5% to 24.1%) and HPV18+ ( n = 5, CIR = 11.8%, 95% CI = 1.9% to 21.7%) women were substantially higher than those for HPV16−/HPV18−/HC2+ ( n = 22, CIR = 3.0%, 95% CI = 1.7% to 4.2%) women and HC2− ( n =46, CIR = 0.8%, 95% CI = 0.5% to 1.0%) women. Although the cumulative risk of ≥CIN3 for women with non-HPV16/18 oncogenic types was relatively low, the overall large number of women with other oncogenic infections produced a substantial number of cases ( n = 22).
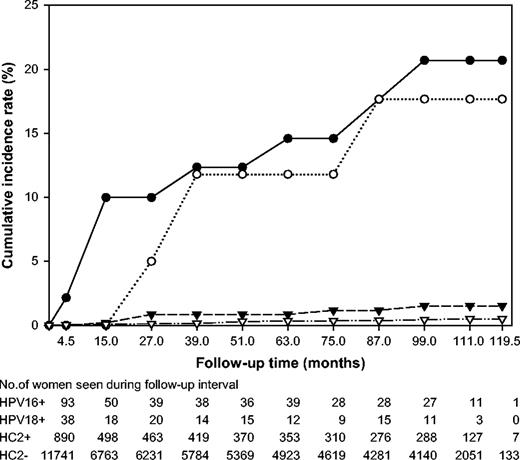
We then focused on women who would be co-tested with HPV and cytology for general screening based on recently published guidelines, i.e., women 30 years of age and older ( 12 – 14 ) . Among the 12 976 women in this group and with negative cytology, the cumulative incidence rates of ≥CIN3 for the HPV groups were as follows: HPV16+, n = 10, CIR = 20.7%, 95% CI = 8.6 to 32.8; HPV18+, n = 3, CIR = 17.7%, 95% CI = 0.0 to 36.0; HPV16−/HPV18−/HC2+, n = 6, CIR = 1.5%, 95% CI = 0.3 to 2.7; and HC2−, n = 26, CIR = 0.5%, 95% CI = 0.3 to 0.7 ( Fig. 3 ).
Cumulative incidence of cervical intraepithelial neoplasia grade 3 and cancer (≥CIN3) over a 10-year period in 12 976 women 30 years old and older with negative cytology at enrollment, according to oncogenic human papillomavirus (HPV) status at enrollment. HPV status is defined hierarchically as: positive for HPV 16 ( closed circles ), else positive for HPV18 ( open circles ), else positive for the non-HPV16/18 oncogenic types in Hybrid Capture 2 (HC2) ( closed triangles ), else oncogenic HPV negative ( open triangles ).
The risks for 471 women with an ASC cytology at enrollment were less clear than the risks for women with negative cytology due to small numbers (data not shown), although HPV16 positivity did appear to confer a higher 10-year risk ( n = 7, CIR = 12.1%, 95% CI = 3.4% to 20.9%) than the other risk groups. In the women with ASC cytology at enrollment, all 20 cases of CIN3 or cancer occurred within the first 2 years after enrollment. Because of very small numbers, the cumulative incidence rates for 124 women with LSIL cytology had wide confidence intervals and therefore could not be reliably interpreted (data not shown).
An examination of the relative contribution of baseline HPV typing and cytology to prospective detection of disease revealed that type-specific HPV testing was a potentially stronger long-term predictor of cervical disease than cytology in women aged 30 years and older ( Table 1 ). A higher cumulative incidence rate of ≥CIN3 was associated with HPV16 positivity among the total group of women with negative, ASC, or LSIL baseline cytology (CIR = 20.1%, 95% CI = 9.7% to 30.6%) than with LSIL cytology among women with HPV-positive or -negative results (CIR = 11.1%, 95% CI = 1.5% to 20.7%). These results revealed that, among women 30 years of age or older, type-specific testing for HPV16 or HPV18 alone had a higher positive predictive value (i.e., number of cases among women with positive tests) than LSIL cytology alone.
The cumulative incidence rates (CIRs) and 95% confidence intervals (CIs) of ≥CIN3 during a 10-year prospective cohort study, according to HPV status and Pap smear diagnosis at enrollment in women ≥30 years old *
| . | CIR (95% CI) by HPV status and Pap smear diagnosis . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| HPV status . | Negative . | ASCs . | LSILs . | Total . | |||
| HPV16+ | 20.7 (8.6 to 32.8) | 7.7 (0.0 to 22.2) | 30.0 (1.6 to 58.4) | 20.1 (9.7 to 30.6) | |||
| HPV18+ | 17.7 (0.0 to 36.0) | 0.0 | 0.0 | 15.4 (0.0 to 31.7) | |||
| Non-HPV16/18 oncogenic+ | 1.5 (0.3 to 2.7) | 6.4 (0.0 to 13.4) | 4.0 (0.0 to 11.7) | 1.8 (0.6 to 3.0) | |||
| Oncogenic HPV− | 0.5 (0.3 to 0.7) | 3.3 (0.1 to 6.6) | 9.1 (0.0 to 26.1) | 0.5 (0.3 to 0.8) | |||
| Total | 0.8 (0.5 to 1.0) | 4.2 (1.3 to 7.1) | 11.1 (1.5 to 20.7) | ||||
| . | CIR (95% CI) by HPV status and Pap smear diagnosis . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| HPV status . | Negative . | ASCs . | LSILs . | Total . | |||
| HPV16+ | 20.7 (8.6 to 32.8) | 7.7 (0.0 to 22.2) | 30.0 (1.6 to 58.4) | 20.1 (9.7 to 30.6) | |||
| HPV18+ | 17.7 (0.0 to 36.0) | 0.0 | 0.0 | 15.4 (0.0 to 31.7) | |||
| Non-HPV16/18 oncogenic+ | 1.5 (0.3 to 2.7) | 6.4 (0.0 to 13.4) | 4.0 (0.0 to 11.7) | 1.8 (0.6 to 3.0) | |||
| Oncogenic HPV− | 0.5 (0.3 to 0.7) | 3.3 (0.1 to 6.6) | 9.1 (0.0 to 26.1) | 0.5 (0.3 to 0.8) | |||
| Total | 0.8 (0.5 to 1.0) | 4.2 (1.3 to 7.1) | 11.1 (1.5 to 20.7) | ||||
A total of 13 229 women aged 30 years and older were tested for HPV status by Hybrid Capture 2. ≥CIN3 = cervical intraepithelial neoplasia grade 3 (CIN3) or cervical cancer; HPV = human papillomavirus; ASC = atypical squamous cell; LSIL = low-grade squamous intraepithelial lesion; oncogenic HPV types = 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. CIRs and 95% CIs were calculated using the Kaplan–Meier method.
The cumulative incidence rates (CIRs) and 95% confidence intervals (CIs) of ≥CIN3 during a 10-year prospective cohort study, according to HPV status and Pap smear diagnosis at enrollment in women ≥30 years old *
| . | CIR (95% CI) by HPV status and Pap smear diagnosis . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| HPV status . | Negative . | ASCs . | LSILs . | Total . | |||
| HPV16+ | 20.7 (8.6 to 32.8) | 7.7 (0.0 to 22.2) | 30.0 (1.6 to 58.4) | 20.1 (9.7 to 30.6) | |||
| HPV18+ | 17.7 (0.0 to 36.0) | 0.0 | 0.0 | 15.4 (0.0 to 31.7) | |||
| Non-HPV16/18 oncogenic+ | 1.5 (0.3 to 2.7) | 6.4 (0.0 to 13.4) | 4.0 (0.0 to 11.7) | 1.8 (0.6 to 3.0) | |||
| Oncogenic HPV− | 0.5 (0.3 to 0.7) | 3.3 (0.1 to 6.6) | 9.1 (0.0 to 26.1) | 0.5 (0.3 to 0.8) | |||
| Total | 0.8 (0.5 to 1.0) | 4.2 (1.3 to 7.1) | 11.1 (1.5 to 20.7) | ||||
| . | CIR (95% CI) by HPV status and Pap smear diagnosis . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| HPV status . | Negative . | ASCs . | LSILs . | Total . | |||
| HPV16+ | 20.7 (8.6 to 32.8) | 7.7 (0.0 to 22.2) | 30.0 (1.6 to 58.4) | 20.1 (9.7 to 30.6) | |||
| HPV18+ | 17.7 (0.0 to 36.0) | 0.0 | 0.0 | 15.4 (0.0 to 31.7) | |||
| Non-HPV16/18 oncogenic+ | 1.5 (0.3 to 2.7) | 6.4 (0.0 to 13.4) | 4.0 (0.0 to 11.7) | 1.8 (0.6 to 3.0) | |||
| Oncogenic HPV− | 0.5 (0.3 to 0.7) | 3.3 (0.1 to 6.6) | 9.1 (0.0 to 26.1) | 0.5 (0.3 to 0.8) | |||
| Total | 0.8 (0.5 to 1.0) | 4.2 (1.3 to 7.1) | 11.1 (1.5 to 20.7) | ||||
A total of 13 229 women aged 30 years and older were tested for HPV status by Hybrid Capture 2. ≥CIN3 = cervical intraepithelial neoplasia grade 3 (CIN3) or cervical cancer; HPV = human papillomavirus; ASC = atypical squamous cell; LSIL = low-grade squamous intraepithelial lesion; oncogenic HPV types = 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. CIRs and 95% CIs were calculated using the Kaplan–Meier method.
To avoid a potential conservative bias, we initially excluded 37 women who tested positive for the 13 oncogenic HPV types (HC2+) but who did not have separate HPV16 and HPV18 typing results. A subanalysis including these women within the HPV16−HPV18−/HC2+ group did not alter our findings (data not shown).
To examine the analytical and clinical sensitivity of the initial HC2 test for detection of HPV16 and HPV18 and clinically relevant infection, we analyzed 1381 HC2− women who also had HPV16 and HPV18 type-specific results. Of these women, only 19 (1.4%) tested positive for HPV16, 5 (0.4%) tested positive for HPV18, and 1 (0.1%) tested positive for both HPV16 and HPV18 by the RNA probes. There were two cases of CIN3 among the 19 women who tested positive for HPV16 by the RNA probes but negative by HC2; these two women also tested positive for HPV16 by MY09/11 polymerase chain reaction (PCR) using type-specific probes, indicating that they were most likely true HPV16 positives who were not detected by HC2. No HC2− women who developed CIN3 or cancer tested positive for HPV18 in this subanalysis.
In another ancillary analysis, we explored the type specificity of the HPV16 and HPV18 RNA probes compared with available MY09/11 PCR data from previously published case–control studies that were conducted during the earlier years of the Kaiser Portland cohort study ( 19 , 27 ) . We did this to examine whether the type-specific probes were cross-reactive with other untargeted HPV types. We found that the single type RNA probes were highly type specific, in that women with other HPV types detected by PCR tested negative (411 of 424 non-HPV16/18 single type infections) for HPV16 and HPV18 using the RNA probes (Supplementary Table 2, available at http://jncicancerspectrum.oxfordjournals.org/jnci/content/vol97/issue14 ). Among women who were HPV16+ by the RNA probes and also had PCR results ( n = 217), there was very little cross-reactivity with other carcinogenic HPV types (3%), and 85% of the infections were confirmed as HPV16+ by PCR (Supplementary Table 3, available at http://jncicancerspectrum.oxfordjournals.org/jnci/content/vol97/issue14 ).
D ISCUSSION
In this cohort study of 20 514 women, the 10-year cumulative incidence rate of CIN3 or cancer was 17% among women who tested positive for HPV16 at enrollment. Among HPV18-positive, non-HPV16/18 oncogenic HPV-positive, and oncogenic HPV-negative women, the 10-year cumulative incidences of ≥CIN3 were 14%, 3%, and 1%, respectively. When we limited the analysis to women aged 30 years and older, for whom HPV testing and co-testing with cytology are an option, the 10-year cumulative incidences of ≥CIN3 among HPV16- and 18-positive women were 20% and 15%, respectively, whereas the 10-year cumulative incidence of ≥CIN3 among women with LSIL cytology at enrollment was 11%.
Recent cervical cancer screening guidelines suggest that oncogenic HPV DNA detection can be usefully introduced into screening of women 30 years of age and older ( 13 ) . However, the large number of cytologically normal women with HPV has led to uncertainty regarding proper follow-up. Interim management guidance to repeat cytologic and HPV DNA screening at 6–12 months was recently proposed because of lack of sufficient data to make a confident decision on the discrete time interval at which follow-up would be appropriate ( 12 ) . We believe that too early repetition of HPV testing would mistakenly characterize as persistent many HPV infections that are destined to resolve. However, a long interval before the follow-up repeat examination can create clinician and patient concern and possible a loss to follow-up.
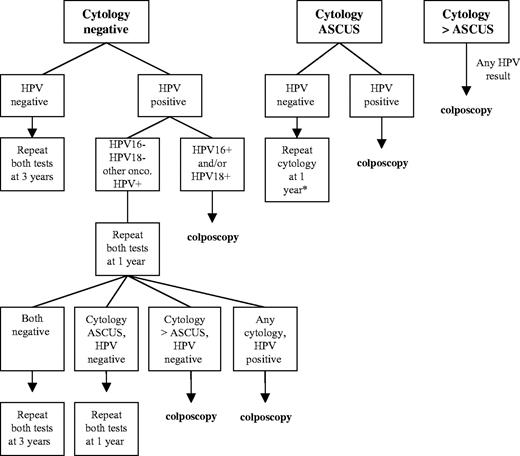
Based on our data, we suggest that separating HPV16 and HPV18, the two most risky oncogenic HPV types, from the other oncogenic HPV types would help to identify, among HPV positive women, the majority destined to progress to ≥CIN3, justifying immediate colposcopy of this subset of infected women and providing reassurance regarding the safety of a 12-month interval without colposcopy if other oncogenic HPV types are detected. Studies have shown that HPV persistence is more likely with HPV16 than with other oncogenic HPV types ( 28 – 31 ) . Women with non-HPV16/18 oncogenic HPV infections will still need to be followed more carefully than women without oncogenic HPV infections, but perhaps more conservatively than women with HPV16 and HPV18 infections. This new screening strategy could help to reduce the number of women who are referred to colposcopy for a positive HPV test ( Fig. 4 ).
Proposed algorithm for the management of women 30 years of age and older in primary cervical cancer screening using a combination of cervical cytology, pooled probe HPV DNA testing, and type-specific HPV16/18 testing. HPV = human papillomavirus; onco = oncogenic; ASCUS = atypical squamous cells of undetermined significance. Adapted from Wright et al. ( 12 ) .
In this prospective cohort study of 20 514 women, 10-year cumulative incidence rates revealed considerably higher risk in women positive for HPV16 or HPV18 at enrollment compared with women positive for non-HPV16/18 oncogenic types and oncogenic HPV-negative women. These findings are consistent with those of other studies in the literature ( 32 , 33 ) and added the strengths of ≥CIN3 outcomes and a more than 20 000-woman prospective study with a large number of older women.
Stratification by age (<30 years versus ≥30 years) demonstrated the high risks associated with HPV16 and HPV18 in both younger and older women. Stratification by enrollment cytology (negative, ASCs, or LSILs) showed that high risks are associated with HPV16 and HPV18 in women with negative cytology; the risks for women with ASCs and LSILs were less clear, owing to small numbers. In particular, the lower rate of ≥CIN3 among women with ASCs in this study (4.2% of women with ASCs at enrollment developed ≥CIN3 over 10 years) compared with another cohort ( 24 ) may be due to the slightly different and possibly lower risk definition of ASCs that we used. An accompanying manuscript by Castle et al. ( 34 ) demonstrated the high risk of CIN3 over a 2-year follow-up associated with HPV16 infection among 5060 women with atypical squamous cells of undetermined significance (ASCUS) or LSILs at enrollment into the ASCUS-LSIL Triage Study; the absence of an elevated risk for HPV18 infection in that study may be the result of an insufficient follow-up period.
Our data ( Table 1 ) suggest that HPV DNA screening of the general population of women aged 30 years and older, with separate typing of HPV16 and HPV18, might be a more powerful predictor of future CIN3 and cancer than ASC or even LSIL cytology. Among women with negative, ASC, and LSIL cytology we observed that a positive HPV16 test alone predicted a higher risk of CIN3 and cancer (20.1%) than a Pap smear with LSIL cytology alone (11.1%). According to current clinical guidelines, any woman with LSIL cytology is referred to colposcopy. Based on our data, it logically follows that women with HPV16 should be referred to colposcopy.
The data in Table 1 touch on a topic under debate by experts in cervical cancer screening—whether cytology or HPV DNA testing should be the primary screening tool for cervical cancer. Although the Pap smear has been used for over 50 years, data from research during the past 20 years has validated the use of HPV testing as an adjunct to primary screening ( 35 ) . Although requiring confirmation in other large screening populations, our prospective results support the notion that cervical cancer screening might gradually turn to a virologic rather than a cytomorphologic paradigm, in which viral type and persistence are key clinical parameters ( 36 ) . However, given that the sensitivity of both methods is imperfect, for situations in which caution is most important, the currently recommended combination of cytology and HPV testing is probably the preferred method.
Our previous study on 20 810 women in the Kaiser Portland cohort, which included women with HSIL cytology at enrollment, showed the 10-year risk of CIN3 or cancer to be approximately 7% for women who tested positive at enrollment by HC2 ( 15 ) . Our present study demonstrates the improvement in positive predictive value that could be achieved with type-specific HPV16 and HPV18 testing adjunctive to a pooled probe HPV test. It is worth considering the possible specific uses of a type-specific test for HPV16 and HPV18 in clinical practice. Women 30 years of age and older could be sampled for cytology, pooled probe HPV DNA testing, and type-specific HPV DNA testing as part of primary screening. Management of cytology and HPV results could proceed as outlined in Fig. 4 . Typing for HPV16 and HPV18 would permit risk stratification of cytologically normal, HPV infected women, a group for whom the length of the repeat screening interval has been unclear. A positive test for HPV16 or HPV18 with any cytology result would warrant referral to colposcopy, whereas cytologically negative women who test positive only for non-HPV16/18 oncogenic types could be retested at 12 months and subsequently referred to colposcopy for repeat LSIL cytology or worse or a repeat positive oncogenic HPV test. Based on current guidelines ( 13 , 14 ) , women who are oncogenic HPV negative with negative cytology can be safely returned to screening every 3 years.
Our study has several limitations. It is likely that our findings in the Kaiser Portland cohort underestimate the true cumulative incidence rates, owing to aggressive management and censoring. Our study was performed in a setting in which participants were screened and treated according to clinical practice that would now be considered aggressive; that is, women were treated at first evidence of CIN2 and, in some cases, CIN1. Treated women were then censored and not followed up further to assess development of ≥CIN3. When we examined the censoring rates among women in our analysis who were not case patients in our analysis, we found that oncogenic HPV-positive women (HPV16+, 12.8% censored; HPV18+, 8.0%; HPV16−/HPV18−/HC2+, 7.0%) were differentially censored ( P <.001) compared with oncogenic HPV-negative women (HC2−, 2.7% censored), although HPV status was not known by clinicians. We presume there would have been many more cases of CIN3 and cancer if this censoring mechanism had not been in place. If so, our calculated estimates of CIR thereby underestimate the true risks associated with HPV16, HPV18, and the other oncogenic HPV types.
Another limitation of our study design was our inability to examine synergy of various oncogenic HPV types. Specifically, we wondered whether inclusion of multiple infections within the HPV16+ group would produce an overestimate of the risks associated with HPV16. The only combination for which we had sufficient HPV typing data to examine possible additive effects was the group of 30 women infected with both HPV16 and 18 at enrollment, three (10.0%) of whom went on to develop ≥CIN3 over 10 years (CIR = 22.3%, 95% CI = 0 to 48.3). This risk did not differ from the risks for all women with HPV16 (CIR = 17.2%) and women with HPV18 without HPV16 (CIR = 13.6%).
Our method of grouping by HPV status assumed that the initial HC2 testing of the entire prospective cohort detected HPV16 and HPV18 infections with reasonable accuracy and with an analytical sensitivity level that was clinically relevant. To test this assumption, we looked at additional available type-specific results using the HPV16 and HPV18 RNA probes in women who had tested negative by HC2 but had HPV infection found by MY09/11 PCR and/or other cervical cancer risk factors. Of these 1381 HC2− women, 1.4%, 0.4%, and 0.1% tested positive for HPV16, HPV18, and both HPV16 and HPV18, respectively, with two cases of ≥CIN3 (3.6% of the 55 HC2− cases in total). Therefore, we believe that HC2 detected the great majority of clinically relevant HPV16 and HPV18 infections and that our HPV typing results are robust.
In conclusion, this prospective Kaiser Permanente cohort study demonstrated that HPV16 and HPV18 are clearly more dangerous than the other oncogenic HPV types, a conclusion consistent with the findings of other cohort studies ( 10 , 11 , 37 ) . Given that HPV16 and HPV18 are estimated from cross-sectional data to cause approximately 70% of cervical cancers worldwide and that the cumulative 10-year risk of ≥CIN3 in women with HPV16 or HPV18 ranges from 10% to 20%, we conclude that these two HPV types are potent carcinogens and should be more effectively targeted in clinical practice. If cost-utility analyses, which are in progress, show single-type tests for HPV16 and HPV18 adjunctive to a pooled HPV test to be cost-effective, then co-testing or triage by HPV16 and HPV18 typing may be a way to focus our clinical attention on a group of HPV-infected women at higher risk for progression to cervical precancer and cancer.
A. Lorincz is the Senior Vice President of Research and Development and Chief Scientific Officer of Digene Corp., the maker of the Hybrid Capture 2 test, and owns stock in the company. M. Sherman has previously received research funding from Digene Corp. D. Scott holds stock in Tripath.
We thank John Schussler, Brian Kramer, and Sabrina Chen of Information Management Services, Inc., for their assistance in data management and analysis.
References
Mahmud SM, Franco EL. An overview of epidemiological and public health research on HPVs presented at the 21st International Papillomavirus Conference in Mexico City, 20–26 February 2004.
Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide.
Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group.
Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer.
IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 90: Human Papillomaviruses. Lyon (France): IARC;
Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis.
Bosch FX, de Sanjose S. Chapter 1: Human papillomavirus and cervical cancer—burden and assessment of causality.
Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica.
Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study.
Winer RL, Kiviat NB, Hughes JP, Adam DE, Lee SK, Kuypers JM, et al. Development and duration of human papillomavirus lesions, after initial infection.
Wright TC Jr, Schiffman M, Solomon D, Cox JT, Garcia F, Goldie S, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening.
Saslow D, Runowicz CD, Solomon D, Moscicki AB, Smith RA, Eyre HJ, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer.
ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists. Number 45, August 2003. Cervical cytology screening (replaces committee opinion 152, March 1995).
Sherman ME, Lorincz AT, Scott DR, Wacholder S, Castle PE, Glass AG, et al. Baseline cytology, human papillomavirus testing, and risk for cervical neoplasia: a 10-year cohort analysis.
Castle PE, Wacholder S, Lorincz AT, Scott DR, Sherman ME, Glass AG, et al. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women.
Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology.
Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study.
Liaw KL, Glass AG, Manos MM, Greer CE, Scott DR, Sherman M, et al. Detection of human papillomavirus DNA in cytologically normal women and subsequent cervical squamous intraepithelial lesions.
Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial.
Schiffman M, Herrero R, Hildesheim A, Sherman ME, Bratti M, Wacholder S, et al. HPV DNA testing in cervical cancer screening: results from women in a high-risk province of Costa Rica.
Lorincz A, Anthony J. Hybrid Capture: A system for nucleic acid detection by signal amplification technology. In: Van Dyke K, Van Dyke C, Woodfork K, editors. Luminescence biotechnology. Boca Raton, FL: CRC Press,
Castle PE, Lorincz AT, Scott DR, Sherman ME, Glass AG, Rush BB, et al. Comparison between prototype hybrid capture 3 and hybrid capture 2 human papillomavirus DNA assays for detection of high-grade cervical intraepithelial neoplasia and cancer.
ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance.
Ferreccio C, Bratti MC, Sherman ME, Herrero R, Hildesheim A, Burk RD, et al. A comparison of single and combined visual, cytologic, and virologic tests as cervical cancer screening methods in a region at high risk of cervical cancer.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations.
Schiffman MH, Bauer HM, Hoover RN, Glass AG, Cadell DM, Rush BB, et al. Epidemiologic evidence showing that human papillomavirus infection causes most cervical intraepithelial neoplasia.
Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica.
Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women.
Wallin KL, Wiklund F, Angstrom T, Bergman F, Stendahl U, Wadell G, et al. Type-specific persistence of human papillomavirus DNA before the development of invasive cervical cancer.
Londesborough P, Ho L, Terry G, Cuzick J, Wheeler C, Singer A. Human papillomavirus genotype as a predictor of persistence and development of high-grade lesions in women with minor cervical abnormalities.
Koutsky LA, Holmes KK, Critchlow CW, Stevens CE, Paavonen J, Beckmann AM, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection.
Cuzick J, Terry G, Ho L, Hollingworth T, Anderson M. Human papillomavirus type 16 in cervical smears as predictor of high-grade cervical intraepithelial neoplasia [corrected].
Castle PE, Solomon D, Schiffman M, Wheeler CM, for the ALTS Group. Human papillomavirus type 16 infections and two-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities.
Wright TC Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ.
Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study.