Abstract
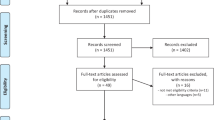
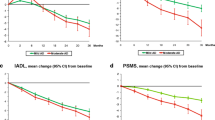
Cholinesterase inhibitors treatment is considered as a common therapeutic approach for Alzheimer’s disease (AD) by numerous reported studies, but the role of currently available drugs for AD is still controversial. Our study aimed to evaluate the efficacy and safety of galantamine for the treatment of AD, and provide the basis and reference for clinical rational drug use. Randomized controlled trials (RCTs) of galantamine for AD published up to April 30, 2014 were searched. A random or fixed-effect model was used to analyze outcomes which were expressed as risk ratios (RRs) or mean difference (MD) with a 95 % confidence interval (CI). Heterogeneity was assessed by Q test and I 2 statistic. The outcome measurements were as follows: the changes of Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), Mini-Mental State Examination (MMSE), Activities of Daily Living (ADL), Neuropsychiatric Inventory (NPI), Clinicians’ Interview-Based Impression of Change with Caregiver’s Input (CIBIC+), adverse effects and dropouts. Eleven articles with 4,074 participants were included. Administration of galantamine for 8–28 weeks (16–40 mg daily) led to significant improvements in ADAS-cog score [P < 0.00001, MD = −2.95, 95 % CI (−3.32, −2.57)], MMSE score [P = 0.003, MD = 2.50, 95 % CI (0.86, 4.15)], NPI score [P = 0.001, MD = −1.58, 95 % CI (−2.54, −0.62)], and CIBIC+ scale [P < 0.00001, RR = 1.26, 95 % CI (1.15, 1.39)], but not in ADL score [P = 0.43, MD = 0.71, 95 % CI (−1.07, 2.48)]. More adverse events and dropouts occurred in the galantamine group than that in the placebo group, the differences were statistically significant (all P < 0.05). Galantamine could significantly improve cognitive, behavioral, and global performances in patients with AD. In addition, we need to use it with caution in the clinical treatment.





Similar content being viewed by others
References
Alzheimer’s Association (2010) 2010 Alzheimer’s disease facts and figures. Alzheimer’s Dement J Alzheimer’s Assoc 6(2):158
American Psychological Association (1994) Diagnostic and statistical manual of mental disorders: DSM-IV. American Psychiatric Association
Birks J (2012) Cholinesterase inhibitors for Alzheimer’s disease (Review)
Bora E, Veznedaroglu B, Kayahan B (2005) The effect of galantamine added to clozapine on cognition of five patients with schizophrenia. Clin Neuropharmacol 28(3):139–141
Brodaty H, Corey-Bloom J, Potocnik FC, Truyen L, Gold M, Damaraju CRV (2005) Galantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer’s disease. Dement Geriatr Cogn Disord 20(2–3):120–132
Burns A, Bernabei R, Bullock R, Jentoft AJC, Frölich L, Hock C, Raivio M, Triau E, Vandewoude M, Wimo A (2009) Safety and efficacy of galantamine (Reminyl) in severe Alzheimer’s disease (the SERAD study): a randomised, placebo-controlled, double-blind trial. Lancet Neurol 8(1):39–47
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10(1):101–129
Gao HZ, Chen Y, Wei Y (2012) Clinical observation on the effect of galantamine in the treatment of mild cognitive impairment. Hainan Medical Journal 23(3):38–39
Hager K, Baseman AS, Nye JS, Brashear HR, Han J, Sano M, Davis B, Richards HM (2014) Effects of galantamine in a 2-year, randomized, placebo-controlled study in Alzheimer’s disease. Neuropsychiatr Dis Treat 10:391–401. doi:10.2147/ndt.s57909
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
Higgins J, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi:10.1136/bmj.d5928
Hohnadel E, Bouchard K, Terry AV Jr (2007) Galantamine and donepezil attenuate pharmacologically induced deficits in prepulse inhibition in rats. Neuropharmacology 52(2):542–551
Hong X, Zhang ZX, Wang LN, Shao FY, Xiao SF, Wang YH, Qian CY, Shu L, Chen SD, Xu XH (2006) A randomized study comparing the effect and safety of galantamine and donepezil in patients with mild to moderate Alzheimer, s disease. Chin J Neurol 39(6):379–382
Inestrosa NC, Sagal JP, Colombres M (2005) Acetylcholinesterase interaction with Alzheimer amyloid β. In: Alzheimer’s disease. Springer US, New York, pp 299–317
Liu FG, Gao ZX, Chen MJ, Ma YX, Shao FY, Zhang GQ, Qu ZW (2003) Evaluation of galantamin in treatment of Alzheimer, s disease: a multicenter, randomized, double-blind study. Chin J New Drugs Clin Rem 22(1):29–32
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ARDA workgroup under the auspices of Department of Health and Human Service Task Force on Alzheimer’s disease. Neurology 34:939–944
Popa RV, Pereira EF, Lopes C, Maelicke A, Albuquerque EX (2006) The N-butylcarbamate derivative of galantamine acts as an allosteric potentiating ligand on α7 nicotinic receptors in hippocampal neurons. J Mol Neurosci 30(1):227–232
Raskind M, Peskind E, Wessel T, Yuan W (2000) Galantamine in AD A 6-month randomized, placebo-controlled trial with a 6-month extension. Neurology 54(12):2261–2268
Raskind MA, Peskind ER, Truyen L, Kershaw P, Damaraju CV (2004) The cognitive benefits of galantamine are sustained for at least 36 months: a long-term extension trial. Arch Neurol 61(2):252–256
Richarz U, Gaudig M, Rettig K, Schauble B (2014) Galantamine treatment in outpatients with mild Alzheimer’s disease. Acta Neurol Scand 129(6):382–392. doi:10.1111/ane.12195
Rockwood K, Mintzer J, Truyen L, Wessel T, Wilkinson D (2001) Effects of a flexible galantamine dose in Alzheimer’s disease: a randomised, controlled trial. J Neurol Neurosurg Psychiatry 71(5):589–595
Samochocki M, Höffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF (2003) Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J Pharmacol Exp Ther 305(3):1024–1036
Sano M, Wilcock GK, van Baelen B, Kavanagh S (2003) The effects of galantamine treatment on caregiver time in Alzheimer’s disease. Int J Geriatr Psychiatry 18(10):942–950
Sasaki H (1996) Clinical feature of dementia of the Alzheimer type. Rinsho Byori Jpn J Clin Pathol 44(3):207–212
Schubert MH, Young KA, Hicks PB (2006) Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol Psychiatry 60(6):530–533
Seltzer B (2010) Galantamine-ER for the treatment of mild-to-moderate Alzheimer’s disease. Clin Interv Aging 5:1
Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC, Tan L (2014) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimer’s Dis: JAD 41(2):615–631. doi:10.3233/jad-132690
Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C (2000) A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology 54(12):2269–2276
Wallin ÅK, Wattmo C, Minthon L (2011) Galantamine treatment in Alzheimer’s disease: response and long-term outcome in a routine clinical setting. Neuropsychiatr Dis Treat 7:565
Wang JH, Wu AQ, Xue SR, Zhang ZC, Zhang BS, Zhan YH (1997) Evaluation of galantamine hydrobromide in treatment of elderly dementia or memory disorders. Acta Academiae Medicinae Suzhou 17(2):310–311
Wang D, Noda Y, Zhou Y, Nitta A, Furukawa H, Nabeshima T (2007) Synergistic effect of galantamine with risperidone on impairment of social interaction in phencyclidine-treated mice as a schizophrenic animal model. Neuropharmacology 52(4):1179–1187
Wilcock GK, Lilienfeld S, Gaens E (2000) Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. BMJ 321(7274):1445
Wilkinson D, Murray J (2001) Galantamine: a randomized, double-blind, dose comparison in patients with Alzheimer’s disease. Int J Geriatr Psychiatry 16(9):852–857
Winblad B, Engedal K, Soininen H, Verhey F, Waldemar G, Wimo A, Wetterholm A-L, Zhang R, Haglund A, Subbiah P (2001) A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57(3):489–495
Xie SZ, Ma YX, Zhu XY, Wu SF, Liu FD, Yang JY, Gu YD (2003) Galantamine in treating age-associated memory impairment and Alzheimer’s disease by double blind method. Geriatr Health Care 9(4):223–226
Acknowledgments
This study was supported by Grants from the National Natural Science Foundation of China (No. 30973162; No. 81302738).
Conflict of interest
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Additional information
D. Jiang and X. Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, D., Yang, X., Li, M. et al. Efficacy and safety of galantamine treatment for patients with Alzheimer’s disease: a meta-analysis of randomized controlled trials. J Neural Transm 122, 1157–1166 (2015). https://doi.org/10.1007/s00702-014-1358-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-014-1358-0