Intended for healthcare professionals
CCBYNC Open access
Rapid response to:
Research
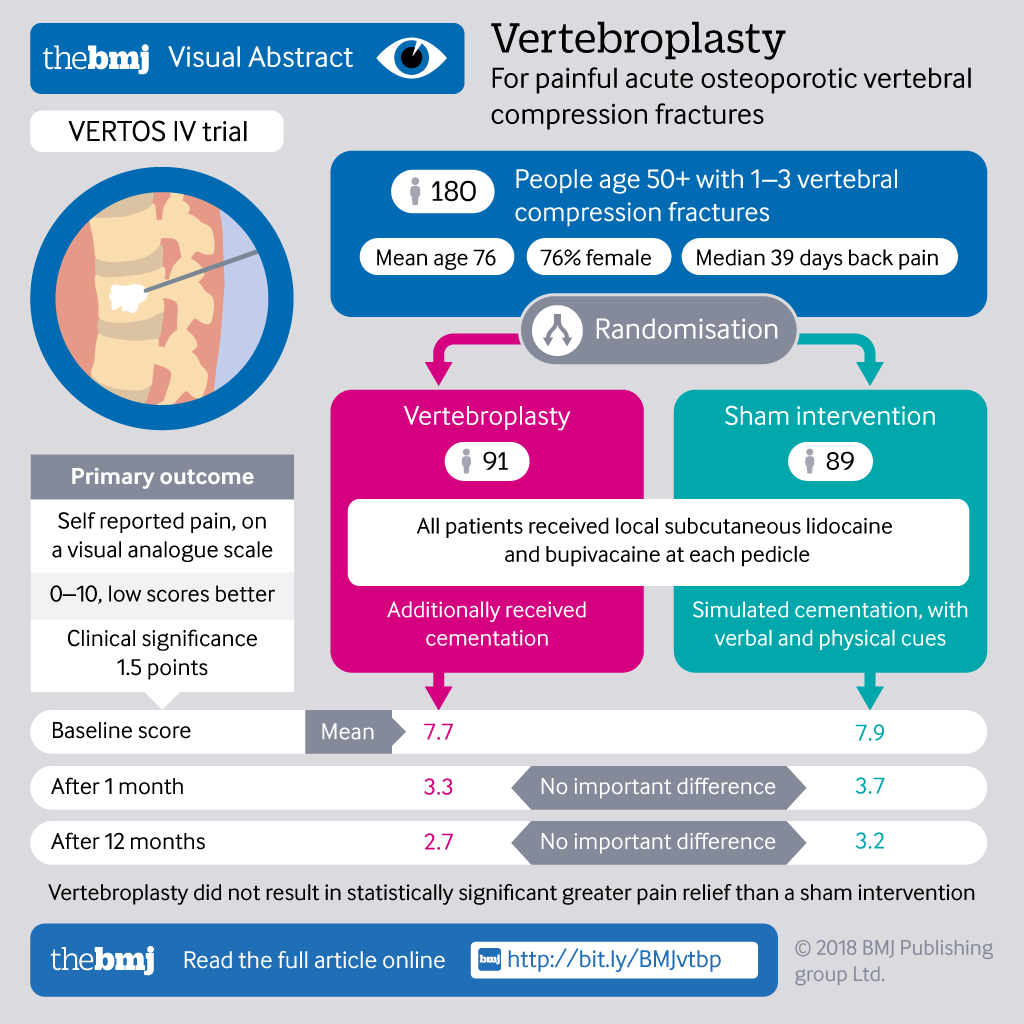
Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial
BMJ 2018; 361 doi: https://doi.org/10.1136/bmj.k1551 (Published 09 May 2018) Cite this as: BMJ 2018;361:k1551
Rapid Response:
Re: Vertebroplasty versus sham procedure for painful acute osteoporotic vertebral compression fractures (VERTOS IV): randomised sham controlled clinical trial
We are concerned by conclusions drawn in the VERTOS IV publication and accompanying editorial.
Randomised clinical trials are designed to assess the efficacy of treatments employed in clinical practice. The conclusions reached are very dependent on the populations recruited and their selection criteria. Patients presenting with acute vertebral fractures may encompass a wide spectrum varying from those with mild to severe osteoporosis, single or multiple comorbidities and those with a pain severity requiring simple analgesia to major opiates. Hospital inpatients are often much worse affected than those who are ambulatory outpatients. It would be an error of judgement to suggest that these cohorts are similar and should be managed equally.
VERTOS4 protocol (1) did not recruit patients with uncontrolled pain referred for vertebroplasty by their attending physicians, but from assessing spinal radiographs performed in 4 radiology centres. Patients with radiographic fractures were given a questionnaire and if back pain was less than 9 weeks duration and at least 5 out of 10 on VAS scale, were invited to participate. Only 34% were using strong opiates and hospitalised inpatients were ineligible. Their osteoporosis could be considered as mild with mean BMD T-scores 0f -2.4 and less than 50% receiving anti-osteoporotic therapies. Patients agreeing to participate were then referred for MRI and physician consult to assess eligibility, causing a mean 14-day delay. At time of vertebroplasty, pain duration was less than 11 weeks, with a median (and mean) of 6.1 weeks. Patient enrolment occurred from April 2011 to April 2014 with final data collection completed three years ago.
Of four blinded, randomised trials of vertebroplasty only the VAPOUR trial (2) has shown clinically important benefits from vertebroplasty. The authors of VERTOS4 acknowledge this but fail to explore the differences between the trials. VAPOUR targeted a different subgroup of patients, who were referred by attending physicians on clinical grounds, with uncontrolled, severe pain, defined as NRS ≥7/10 despite maximal medical therapy, including opiates. 57% were hospital inpatients and 43% outpatients and all had severe osteoporosis. Vertebroplasty was offered without further delay, to control the acute pain syndrome, facilitate rapid rehabilitation and prevent the downhill spiral so often seen in the elderly with catastrophic spinal fractures. Most patients (80%) underwent vertebroplasty within 3 weeks of fracture onset (mean fracture duration 2.6 weeks compared to 6.1 weeks in VERTOS4).
Failure of medical therapy to adequately control acute fracture pain indicated poor outcomes from placebo therapy in VAPOUR. Most placebo group patients still had moderate or severe pain at 6 months and 76% were still using analgesics, whereas the vertebroplasty group derived clinically important benefits at all time points. Early intervention makes vertebroplasty technically easier, as the fracture is soft and physically compliant, accepting a larger PMMA volume (7.5cc in VAPOUR vs 5.1cc in VERTOS4) with a reduced PMMA leak rate (34% in VAPOUR vs 91% in VERTOS4). It also facilitated vertebral height restoration. The very high leak rate in VERTOS4 suggests that the vertebral body had already consolidated.
Patients with severe pain (NRS ≥7/10) due to osteoporotic spinal fracture less than 6 weeks duration should be offered maximal medical therapy including opiate analgesia. If this fails to adequately control the acute pain syndrome, or if the patient cannot tolerate the opiates due to side effects, then they should be offered early vertebroplasty without further delay. This includes the most severely affected outpatients and hospital inpatients. This reinforces the notion that scientific evidence is an essential part of advancing medical knowledge, but it must be integrated with clinical experience to complete the process. Only then can we inform decision making in clinical care and deliver the best clinical outcomes available.
1. Firanescu C, Lohle PN, de Vries J, et al. A randomised sham controlled trial of vertebroplasty for painful acute osteoporotic vertebral fractures (VERTOS IV). Trials. 2011;12:93. doi:10.1186/1745-6215-12-93.
2. Clark, W, Bird, P, Gonski, P et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fracture (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1408–16. doi.org/10.1016/S0140-6736(16)31341-1
Competing interests: We are authors of the VAPOUR trial.