Emollient bath additives for the treatment of childhood eczema (BATHE): multicentre pragmatic parallel group randomised controlled trial of clinical and cost effectiveness
BMJ 2018; 361 doi: https://doi.org/10.1136/bmj.k1332 (Published 03 May 2018) Cite this as: BMJ 2018;361:k1332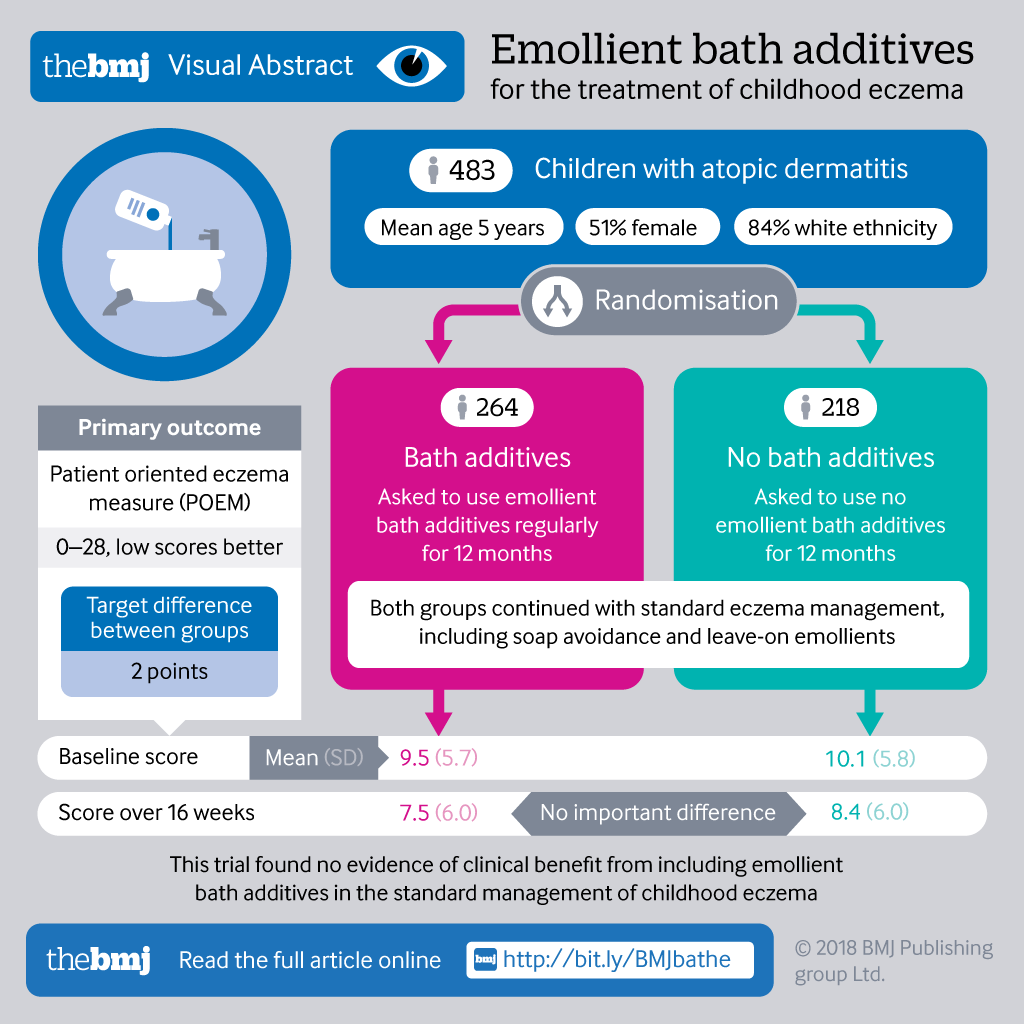
Linked Editorial
New evidence challenges use of bath emollients for children with eczema
Opinion: Miriam Santer
Patient and carer choice for eczema treatment is crucial
- Status
- Comments
- Date
- Original article
- Access document
- 13 December 2017
- First decision
- Access document
- 07 February 2018
- Author response
- Access document
- 24 February 2018
- First revised article
- Access document
- 24 February 2018
- Second decision
- Access Document
- 12 March 2018
- Second author response
- Access Document
- 13 March 2018
For research papers The BMJ has fully open peer review. This means that accepted research papers published from early 2015 onwards usually have their prepublication history posted alongside them on bmj.com.
This prepublication history comprises all previous versions of the manuscript, the study protocol (submitting the protocol is mandatory for all clinical trials and encouraged for all other studies at The BMJ), the report from the manuscript committee meeting, the reviewers’ comments, and the authors’ responses to all the comments from reviewers and editors.
In rare instances we determine after careful consideration that we should not make certain portions of the prepublication record publicly available. For example, in cases of stigmatised illnesses we seek to protect the confidentiality of reviewers who have these illnesses. In other instances there may be legal or regulatory considerations that make it inadvisable or impermissible to make available certain parts of the prepublication record.
In all instances in which we have determined that elements of the prepublication record should not be made publicly available, we expect that authors will respect these decisions and also will not share this information.
