Article Text
Abstract
Objective: To explore the causes of preventable drug-related admissions (PDRAs) to hospital.
Design: Qualitative case studies using semi-structured interviews and medical record review; data analysed using a framework derived from Reason’s model of organisational accidents and cascade analysis.
Participants: 62 participants, including 18 patients, 8 informal carers, 17 general practitioners, 12 community pharmacists, 3 practice nurses and 4 other members of healthcare staff, involved in events leading up to the patients’ hospital admissions.
Setting: Nottingham, UK.
Results: PDRAs are associated with problems at multiple stages in the medication use process, including prescribing, dispensing, administration, monitoring and help seeking. The main causes of these problems are communication failures (between patients and healthcare professionals and different groups of healthcare professionals) and knowledge gaps (about drugs and patients’ medical and medication histories). The causes of PDRAs are similar irrespective of whether the hospital admission is associated with a prescribing, monitoring or patient adherence problem.
Conclusions: The causes of PDRAs are multifaceted and complex. Technical solutions to PDRAs will need to take account of this complexity and are unlikely to be sufficient on their own. Interventions targeting the human causes of PDRAs are also necessary—for example, improving methods of communication.
Statistics from Altmetric.com
Preventable drug-related morbidity (PDRM) is an important public health issue which accounts for over 4% of admissions to hospital,1 and injuries in up to 3% of patients in their own home.2 PDRM is often linked to errors in the medication use process, which continue to be an important policy issue. For example, the World Health Organization is currently organising a patient safety initiative in seven countries seeking to impact on patient safety problems (including medication errors).3 To be successful, a “systems” approach, understanding the “root causes”, is probably needed, and this will require an understanding of the complexity of the causes of medication errors.4 Currently, those studies exploring the causes of medication errors are largely confined to secondary care5–13 and those that have explored medication errors in primary care have largely concentrated on discrete stages of the medication use process (eg, prescribing errors14 or dispensing errors15) without examining how errors occur in complex systems.16 Furthermore, those studies which have explored medication errors or PDRM in primary care have typically relied on retrospective documentary data.1718 As a result, relatively little is known about the causes of medication errors in primary care which result in serious patient harm, such as hospitalisation. In addition, few studies have used a qualitative approach to understand PDRM and very few have explored the role of patients in relation to PDRM.19
The present study used a qualitative approach that included interviews with patients, general practitioners (GPs) and community pharmacists, and reviews of medical records and policies, to explore the causes of PDRM leading to hospitalisation. We adapted Reason’s model of organisational accidents20 and cascade analysis21 as an analytic framework for our study (fig 1). Studying the cascade of events which lead to PDRM provides a more complete picture of the causes of errors within a complex health system and also highlights the range of safety barriers that need to be overcome.
METHODS
We studied preventable drug-related admissions (PDRAs) to a teaching hospital in Nottingham, UK, originating from four primary care trusts (PCTs), between 1 January and 31 December 2004. The Nottingham research ethics committee and research governance offices approved the study and all participants gave written informed consent. The methods used for case identification and assessment22 and healthcare professional recruitment23 are described in detail elsewhere. All methods are described in brief below.
Recruitment
In November 2003 consent was requested from all GPs in four Nottingham PCTs to recruit and interview patients registered at their practices in the event that a PDRA was identified during the study period. During 2004, clinical ward pharmacists screened patients admitted to medical, care of the elderly and neurology wards at Queen’s Medical Centre, Nottingham, for possible drug-related causes of their hospital admission. Only patients with admissions associated with the most common drug causes of PDRAs122 (table 1), and whose GPs had consented to participate in the study, were approached. RH then sought written informed consent from these patients to be interviewed, and for permission to contact their GP and community pharmacist.
RH assembled detailed case summaries of events leading to hospital admissions from short interviews and medical record reviews. Using previously validated methods, case summaries were assessed for causality and preventability.22 Patients whose cause of admission was considered to be drug-related and preventable were included in the study.
RH asked patients to identify which GP and community pharmacist they most commonly saw. These healthcare professionals were then contacted to request their participation in the study.
Data collection
Following patient discharge, RH undertook semi-structured, audiotaped interviews with patients (in their home), GPs (in their surgery), and community pharmacists (in their pharmacy). Consent was reconfirmed verbally before each interview. Additional healthcare professionals involved in the patient’s care and medication provision were identified from participants and interviewed at their place of work (these included nurses, practice receptionists and a practice manager). The interview schedule was developed following discussion with experts on medication safety and following pilot work. The interview schedule varied according to the person interviewed. See box 1 for an overview of the topics included in the patient, GP and pharmacist interviews. Also, relevant information was abstracted from hospital, GP and community pharmacist patient records, policies and procedures.
Box 1: Topics included in the interview schedules with patients, GPs and pharmacists
Interviews with patients
Details of events leading up to hospital admission
Knowledge of cause of hospital admission
Patients’ perception of preventability of hospital admission
Knowledge about medication
How medication was managed at home
Sources of information about medication
Relationship with healthcare professionals
Interviews with GPs
Knowledge of cause of hospital admission
GP’s involvement in events leading up to hospital admission
Case-specific questions about medication knowledge
Factors influencing decisions/performance during these events
GP’s perception of preventability of hospital admission
Relationship with patient
GP’s perceptions about the patient and the patient’s level of understanding
Impact of PDRA on the GP and their future practice
Awareness and perspectives on systems of medicines management within the practice
GP’s previous experience of adverse drug events in their patients
Interviews with pharmacists
Knowledge of cause of hospital admission
Pharmacist’s involvement in events leading up to hospital admission
Case-specific questions about medication knowledge
Factors influencing decisions/performance during these events
Pharmacist’s perception of preventability of hospital admission
Relationship with patient
Pharmacist’s perceptions about the patient and the patient’s level of understanding
Impact of PDRA on the pharmacist and their future practice
Awareness and perspectives on systems of medicines management within the pharmacy
Pharmacist’s previous experience of adverse drug events in their patients
Analytic framework
Reason’s model of organisational accidents has been used as a framework for analysing adverse events and errors5 but this model can fragment the understanding of contributing factors by separating them into groups (eg, environment, team, task, patient and individual factors). Woolf et al21 combined Reason’s model of organisational accidents and cascade theory to develop an alternative analytic model that depicts how contributing factors and errors (active failures) cascade into one another, resulting in adverse events. We adapted these models to produce an analytic framework for the present study based on real-life cases of PDRAs (see fig 1).
Data processing and analysis
Audiotapes of interviews were transcribed verbatim. RH checked the transcripts for accuracy against the original recordings and removed identifying references to individuals. To analyse the data, RH firstly used iterative readings of interview transcripts and documentary data to develop timelines and case synopses describing the sequence of events leading up to patients’ admissions and the key factors contributing to these admissions. RH then used iterative readings of case synopses and template analysis24 to develop error cascade diagrams for each case (see fig 1). In template analysis, higher level codes are determined a priori and form a template for analysis which is then modified and added to as the analysis progresses.
The error cascades produced for individual cases summarised the contributing factors, proximal causes (defined by Leape et al8 as “the apparent reason an error was made”), active failures, and safety barriers overcome in events leading up to each patient’s admission (examples of these cascade diagrams are available online as supplementary error cascades). Across-case analysis25 was used to develop a cascade diagram incorporating the main features of all cases (fig 2). Each stage of the analytic process was discussed in detail by RH, PB and TA to reduce individual biases.
RESULTS
Recruitment
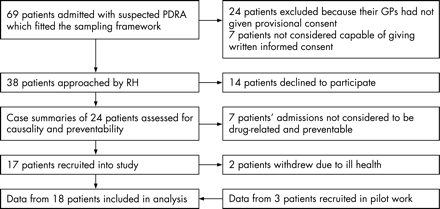
We recruited 15 patients into the study from 38 potentially eligible patients identified during the study period (7 patients’ admissions were not considered to be PDRA, 14 declined to participate and 2 withdrew due to ill health). Data from three more patients recruited during pilot work were included in the analysis (fig 3). RH conducted interviews with 17 patients (median duration 45 min, range 25–90), 17 GPs (median 22.5 min, range 10–65), 12 community pharmacists (median 45 min, range 20–60), 3 practice nurses, 2 senior receptionists, 1 practice manager and 1 hospital pharmacist (median 20 min, range 5–75). During interviews with patients, the main carer contributed to the interviews in eight cases. One patient was not interviewed because they died shortly after discharge to home. In one case the GP was not interviewed because the drug causing admission had been purchased from a community pharmacy. In six cases the community pharmacists had not given prior agreement to be interviewed. Demographic characteristics of the interviewees and the causes of patients’ admissions are available in the online supplementary tables.
Causes of preventable drug-related hospital admissions
The contributing factors, proximal causes and active failures which led to the PDRAs are illustrated in fig 2. The numbers in parentheses indicate the number of cases in which each problem was found (not every contributing factor, proximal cause and active failure occurred in each case). Figure 2 illustrates the complexity of the events which can contribute to a PDRA. The cases involved multiple active failures (median 4, range 3–7) cascading together to result in a PDRA. Active failures were observed at all stages of the medication use process (prescribing, dispensing, administering and monitoring medication, and patients seeking help for problems with medication). In each case, active failures occurred at multiple stages in the medication use process and multiple defences against each active failure were overcome, including the potential roles of patients, carers, and healthcare professionals (community pharmacists, GPs, practice nurses, practice receptionists, hospital doctors, hospital nurses, hospital pharmacists and pharmacy technicians). The computer systems used by GPs and community pharmacists also failed to prevent PDRAs, and in some cases acted as contributing factors. The most prominent proximal causes of PDRAs were:
communication problems between patients and healthcare professionals, GPs and pharmacists, and GPs and hospital doctors;
knowledge gaps about medications;
knowledge gaps about patients’ medical and medication histories.
These are described below in more detail and illustrative quotes (referred to in the text by the interviewee’s identification code) are shown in table 2.
Communication problems
Insufficient patient counselling about medication was an important communication problem between patients and healthcare professionals. Many patients indicated that they were reluctant to question healthcare professionals, especially GPs, about their medication (see Pt25, table 2). Some community pharmacists assumed that patients would have received medication counselling from the GP or other healthcare professional when obtaining their prescription and so did not counsel patients themselves. Some community pharmacists also did not perceive medication counselling to be their role (see Phrm5, table 2). Some patients indicated that they could not recall information which they had been given, or had difficulty hearing the information healthcare professionals gave (see Pt15, table 2). These important issues relating to communication underpinned patients’ knowledge gaps about medication, resulting in active failures by patients and carers in appropriately administering medication, monitoring medication, and seeking help for problems with medication (such as how to take the medication or how to respond to potential adverse effects).
Communication problems between GPs and community pharmacists were often associated with the asymmetrical relationships between these professional groups. Although some community pharmacists recognised that prescriptions were potentially harmful, they indicated reluctance to question GPs because they had insufficient information about patients’ medical and medication history. Previous negative experiences of raising problems with GPs, and the time taken to contact GPs, contributed to this reluctance (see Phrm20, table 2).
Delays in sending outpatient letters and the limited information in discharge letters restricted communication between GPs and hospital doctors. Delayed letters meant that information was unavailable when needed (see Case27, table 2) and incomplete information on discharge letters meant GPs sometimes had insufficient information to safely manage medication started by hospital doctors (see GP12, table 2).
Knowledge gaps
Difficulties in accessing complex medical and medication histories in electronic patient records were associated with gaps in GP knowledge. Information about important risk factors for adverse events was easily lost in long electronic records and failure to update medical records following home visits meant relevant information was sometimes missing (see GP21, table 2). GPs were not alerted to some high-risk prescriptions because computer systems did not link patient diagnoses and blood test results to prescribed medication (see GP27, table 2).
Community pharmacists lacked access to patients’ medical records (see Phrm27, table 2) and medication records held in pharmacies could be incomplete because patients attended more than one community pharmacy. Where medication histories were available, some computer systems provided non-specific alerts to potential interactions that did not provide sufficient information (see Phrm25, table 2). These factors made clinical screening of prescriptions extremely difficult.
GPs and community pharmacists sometimes had insufficient knowledge of the medication they prescribed, dispensed or monitored (see Phrm6, table 2). This lack of knowledge was exacerbated because computer systems did not alert to potential medication problems (see GP27, table 2) and available reference sources were not consulted. Some professionals assumed that they knew enough to safely manage the medication without consulting reference sources (see GP5, table 2). In other cases, there was inadequate information about how and when to monitor medication from secondary care (see GP12, table 2).
DISCUSSION
Our analysis suggests that the causes of PDRAs are multifaceted and complex. Active failures occur at multiple stages in the medication use process in each case. The major proximal causes of PDRA are communication failures and knowledge gaps, underpinned by a variety of contributing factors, including time and workload pressures and problems with computer system design.
Strengths and weaknesses of the study
We undertook a prospective, detailed exploration of a small number of cases of PDRA and have gained an indepth understanding of the causes of the most common drug causes of these admissions (see table 1). By using prospective interview data and documentary review we have been able to produce a reasonably complete data set for each PDRA. We have enhanced the validity of the data collected by triangulating the perspective of three key players in events leading up to PDRA. Furthermore, we have addressed issues to do with analytic validity through discussion of our individual data interpretations at each stage of the analytic process. Our approach has overcome some of the problems with retrospective analysis of adverse event reports and claims data, which is often limited by fragmentation of records, missing data and inability to verify data.2627 Compared with most retrospective studies, however, we have explored a relatively small number of cases, but this has enabled us to undertake an indepth analysis of their causes. This limits the generalisability of our data. All data were collected within the Nottingham area which may not be representative of primary care across the rest of the UK. The cases were, however, purposively selected to ensure they were representative of the most common drug causes of PDRA. These drug causes are known to occur throughout the UK. Therefore, it is likely that our data will be applicable to the provision of primary care throughout the UK.
Previous studies which have used human factors theory as an analytic framework have not conveyed a sense of the complexity of the causes of medication errors and PDRM.578 Combining cascade theory (see figs 1 and 2) and human factors theory in our analytic framework allowed us to focus on the causes of PDRA while retaining an understanding of the complexity of the interactions between different aspects of the medication use process. Also, by studying PDRA, rather than errors at specific stages in the medication use process, we have illustrated how errors at each stage cascade into each other, resulting in patient harm. In fig 2 we have illustrated how each PDRA involved errors at almost every stage in the medication use process. This reinforces the need for interventions at multiple stages in the medication use process.28
In common with previous studies of adverse events and errors, we found communication problems and knowledge gaps to be the major proximal causes of PDRA.56821 In contrast with these studies, our focus on primary care and our inclusion of patients’ perspectives has helped to identify communication problems between patients and healthcare professionals, and patients’ knowledge gaps about medication, as major proximal causes of errors and PDRA (this is in addition to communication problems between different healthcare professional groups and healthcare professionals’ knowledge gaps about medication). This has not been found in studies which have concentrated on adverse events from the healthcare professional’s perspective. The relative dearth of patient involvement and patient perspectives in the patient safety movement has been highlighted by Vincent and Coulter.19 Our study begins to elicit the important role of patients in the patient safety arena.
Implications of findings for practice and future research
This study has also highlighted the importance of communication problems and knowledge gaps in PDRM, and our findings highlight possible targets for future interventions to reduce PDRM. Technical solutions are likely to be useful, but need to take account of the complexity of the causes of PDRM. For example, enhanced decision support for computerised prescribing is likely to help reduce errors at the prescribing stage by providing prescribers with easy access to information on patients’ comorbidities and the risks associated with prescribing specific medications.29 Decision support could also assist prescribers in the appropriate monitoring of medication.30
The NHS patient care record, under development in the UK, could help to alleviate some of the communication problems seen in this study.31 If implemented effectively, it would allow prescribers rapid access to medication and medical histories when patients are transferred between primary and secondary care, as well as the results of monitoring.
In secondary care, pharmacists are recognised as an important patient safety resource.32 Hospital pharmacists are assisted in this role by easy access to medical records. At present, community pharmacists do not have access to this information and their role in patient safety is more limited. The NHS patient care record could give them access to the medical and medication histories which they need to be able to function as adequate defences against PDRM. Once community pharmacists have this information, however, they are likely to need additional training to ensure they can use it effectively.33 More work will also be needed to address asymmetrical relationships between pharmacists and prescribers to make it easier for pharmacists to question potential problems they find on prescriptions.34
To maximise patients’ ability to manage their own medication safely and appropriately, interventions are needed which provide adequate information and help to ensure patients’ understanding. Interventions in which prescribers encourage patients to talk about their medicines in consultations have been successful in increasing patients’ medication knowledge, but the impact on patients’ outcomes is unknown.35 Furthermore, interventions which address the asymmetrical relationships between patients and prescribers may be helpful in facilitating effective discussion about medication. One solution could be providing additional communication training to healthcare professionals, including feedback.
We have identified two major proximal causes of PDRAs. More studies are needed to identify strategies to help overcome these problems and improve patient outcomes. The complexity of the causes of PDRAs makes it unlikely that single interventions targeted at one stage in the medication use process will be sufficient to substantially reduce PDRM. Instead, successful interventions will need to be multifaceted.
Acknowledgments
RH was employed by the Nottingham Primary Care Research Partnership at the time of the study. PB was employed by the University of Nottingham at the time of the study.
REFERENCES
Footnotes
Funding: Funded by NHS R&D funding, through the Nottingham Primary Care Research Partnership, and £10 000 Galen award from the Pharmacy Practice Research Trust. The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. RH had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests: None.
Ethics approval: The Nottingham research ethics committee and research governance offices approved the study.
Linked Articles
- Quality lines