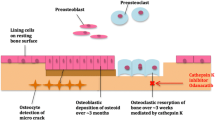
Abstract
Osteoporosis is a systemic bone disease characterized by low bone mass and bone mineral density, and deterioration of the underlying structure of bone tissue. These changes lead to an increase in bone fragility and an increased risk for fracture, which are the clinical consequences of osteoporosis. The classical triad for consideration in osteoporosis is morbidity, mortality and cost. Vertebral fracture is an important source of morbidity in terms of pain and spinal deformity. On the other hand, hip fracture is associated with the worst outcomes and is widely regarded as a life-threatening event in the elderly; it is the source of the bulk of the cost of the disease in contemporary healthcare.
The prevention of osteoporosis-associated fracture should include fall prevention, calcium supplementation and lifestyle advice, as well as pharmacological therapy using agents with proven antifracture efficacy. The most commonly used osteoporosis treatments in Europe are the bisphosphonates alendronate, risedronate, ibandronate and zoledronic acid; the selective estrogen receptor modulator (SERM) raloxifene; teriparatide; and strontium ranelate. Recent additions include the biological therapy denosumab and the SERM bazedoxifene. In this review, we explore the antifracture efficacy of these agents with the aim of simplifying treatment decisions. These treatments can be broadly divided according to their mode of action. The antiresorptive agents include the bisphosphonates, the SERMs and denosumab, while the bone-forming agents include parathyroid hormone and teriparatide. Strontium ranelate appears to combine both antiresorptive and anabolic activities. We collated data on vertebral and hip fracture efficacy from the pivotal 3-year phase III trials, all of which had a randomized, double-blind, placebo-controlled design. The relative reductions in risk in the osteoporosis trials range from 30% to 70% for vertebral fracture and 30% to 51% for hip fracture. This translates into 3-year number needed to treat values of between 9 and 21 for vertebral fracture and from 48 upwards for hip fracture.
International guidelines agree that agents that have been shown to decrease vertebral, nonvertebral and hip fractures should be used preferentially over agents that only demonstrate vertebral antifracture efficacy. This is the case for alendronate, risedronate, zoledronic acid, denosumab and strontium ranelate. Finally, therapeutic decisions should be based on a balance between benefits and risks of treatment, which must be carefully considered in each particular case both by the physician and the patient. Indeed, no single agent is appropriate for all patients and, therefore, treatment decisions should be made on an individual basis, taking into account all measures of treatment effect and risk before making informed judgments about the best individual treatment option.




Similar content being viewed by others
References
Kanis J, Burlet N, Cooper C, et al. European Guidance for the diagnosis and treatment of osteoporosis in postmeno-pausal women. Osteoporos Int 2008 Apr; 19(4): 399–428
Reginster J-Y, Rizzoli R. Innovation in skeletal medicine. In: Reginster J-Y, Rizzoli R, editors. Innovation in skeletal medicine. Issy-les-Moulineaux: Elsevier, 2007: 7–9
Kanis JA, on behalf of the World Health Organization Scientific Group. Assessment of osteoporosis at the primary healthcare level. Technical report. Sheffield: WHO Centre for Metabolic Bone Diseases, 2007: 38
Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fracture with back pain and function: a prospective study. Ann Intern Med 1998 May; 128(10): 793–800
Sambrook P, Cooper C. Osteoporosis. Lancet 2006 Jun; 367(9527): 2010–8
Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001 Jan; 285(3): 320–3
Center JR, Bliuc D, Nguyen TV, et al. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007 Jan; 297(4): 387–94
Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004 Aug; 35(2): 375–82
Cooper C, Campion G, Melton 3rd LJ. Hip fractures in the elderly: a world-wide projection. Osteoporos Int 1992 Nov; 2(6): 285–9
Rizzoli R, Bruyere O, Cannata-Andia JB, et al. Management of osteoporosis in the elderly. Curr Med Res Opin 2009 Oct; 25(10): 2373–87
Boonen S, Singer AJ. Osteoporosis management: impact of fracture type on cost and quality of life in patients at risk for fracture. Curr Med Res Opin 2008 Jun; 24(6): 1781–8
Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ 2009 Sep; 181(5): 265–71
Cauley J, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int 2000; 11(7): 556–61
Leibson CL, Tosteson AN, Gabriel SE, et al. Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc 2002 Oct; 50(10): 1644–50
Richmond J, Aharonoff GB, Zuckerman JD, et al. Mortality risk after hip fracture. J Orthop Trauma 2003 Jan; 17(1): 53–6
Empana JP, Dargent-Molina P, Breart G. Effect of hip fracture on mortality in elderly women: the EPIDOS prospective study. J Am Geriatr 2004 May; 52(5): 685–90
Abrahamsen B, van Staa T, Ariely R, et al. Excess mortality following hip fracture: a systematic epidemiological review. Osteoporos Int 2009 Oct; 20(10): 1633–50
Salkeld G, Cameron ID, Cumming RG, et al. Quality of life related to fear of falling and hip fracture in older women: a time trade off study. BMJ 2000 Feb; 320(7231): 341–6
Rabenda V, Manette C, Lemmens R, et al. The direct and indirect costs of the chronic management of osteoporosis: a prospective follow-up of 3440 active subjects. Osteoporos Int 2006; 17(9): 1346–52
International Osteoporosis Foundation. Osteoporosis in the European Community: a call to action. An audit of policy developments since 1998 [online]. Available from URL: http://www.iofbonehealth.org/download/osteofound/filemanager/publications/pdf/eu-report-2001.pdf [Accessed 2010 Sep 1]
Reginster JY, Burlet N. Osteoporosis: a still increasing prevalence. Bone 2006 Feb; 38 (2 Suppl. 1): S4–9
International Osteoporosis Foundation. Osteoporosis in the European Union in 2008: ten years of progress and ongoing challenges [online]. Available from URL: http://www.sante.public.lu/publications/maladies-traitements/osteoporose/osteoporosis-eu-2008/osteoporosis-eu-2008.pdf [Accessed 2010 Sep 1]
Body JJ, Bergmann P, Boonen S, et al. Evidence-based guidelines for the pharmacological treatment of postmenopausal osteoporosis: a consensus document by the Belgian Bone Club. Osteoporos Int 2010 Oct; 21(10): 1657–80
Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 2008 Sep; 83(9): 1032–45
Delmas PD. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom 2008 Apr–Jun; 11(2): 325–38
Cummings SR, San Martin J, McClung MR, et al. Deno-sumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009 Aug; 361(8): 756–65
Reid I, Miller P, Brown J, et al., on behalf of the Denosumab Phase 3 Bone Histology Study Group. Effects of denosumab on bone histomorphometry: the freedom and stand studies. J Bone Miner Res 2010 Oct; 25(10): 2256–65
Riggs BL, Hartmann LC. Selective estrogen-receptor modulators: mechanisms of action and application to clinical practice. N Engl J Med 2003 Feb; 348(7): 618–29
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001 May; 344(19): 1434–41
Whitfield JF, Morley P, Willick GE. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treat Endocrinol 2002; 1(3): 175–90
Reeve J. Recombinant human parathyroid hormone. BMJ 2002 May; 324(7347): 435–6
Marie PJ. Strontium ranelate: a dual mode of action rebalancing bone turnover in favour of bone formation. Curr Opin Rheumatol 2006 Jun; 18 Suppl. 1: S1 1–5
Reginster JY, Deroisy R, Neuprez A, et al. Strontium ranelate: new data on fracture prevention and mechanisms of action. Curr Osteoporos Rep 2009 Sep; 7(3): 96–102
Deeks ED, Dhillon S. Strontium ranelate: a review of its use in the treatment of postmenopausal osteoporosis. Drugs 2010 Apr; 70(6): 733–59
Song F, Loke YK, Walsh T, et al. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ 2009 Apr; 338: b1 147
Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet 1996 Dec; 348(9041): 1535–41
Chesnut III CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004 Aug; 19(8): 1241–9
Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999 Oct; 282(14): 1344–52
Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 2000; 11(1): 83–91
Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007 May; 356(18): 1809–22
Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 1999 Aug; 282(7): 637–45
Cummings SR, Ensrud K, Delmas PD, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 2010 Feb; 362(8): 686–96
Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 2008 Dec; 23(12): 1923–34
Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 2004 Jan; 350(5): 459–68
McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women: Hip Intervention Program Study Group. N Engl J Med 2001 Feb; 344(5): 333–40
Reginster JY, Seeman E, De Vernejoul MC, et al. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: TROPOS study. J Clin Endocrinol Metab 2005 May; 90(5): 2816–22
MacLaughlin EJ, Raehl CL. ASHP therapeutic position statement on the prevention and treatment of osteoporosis in adults. Am J Health Syst Pharm 2008 Sep; 65(3): 343–57
Sackett DL, Straus SE, Richardson WS, et al., editors. Evidence-based medicine: how to practice and teach EBM. 2nd ed. Edinburgh: Churchill Livingstone, 2001
Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010 Mar; 340: c8609
Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010 Mar; 340: c332
Gallagher JC, Genant HK, Crans GG, et al. Teriparatide reduces the fracture risk associated with increasing number and severity of osteoporotic fractures. J Clin Endocrinol Metab 2005 Mar; 90(3): 1583–7
Agence française de sécurite sanitaire des produits de santé. Traitement medicamenteux de l’ ostéoporose post-méno-pausique, 2006 [online]. Available from URL: http://www.afssaps.fr/Infos-de-securite/Recommandations-de-bonne-pratique/Traitement-medicamenteux-de-l-osteoporose-post-menopausique-recommandations-de-bonne-pratique/(language)/fre-FR [Accessed 2010 Sep 1]
Committee for Medicinal Products for Human Use (CHMP). Guideline on the evaluation of medicinal products in the treatment of primary osteoporosis. Ref CPMP/EWP/552/95Rev.2. London: CHMP, 2006
Kanis JA, McCloskey EV, Johansson H, et al. Development and use of FRAX in osteoporosis. Osteoporos Int 2010 Jun; 21 Suppl. 2: S407–13
Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008 Apr; 19(4): 385–97
Abrahamsen B. Adverse effects of bisphosphonates. Calcif Tissue Int 2010 Jun; 86(6): 421–35
Musette P, Brandi ML, Cacoub P, et al. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int 2010 May; 21(5): 723–32
Brennan TC, Rizzoli R, Ammann P. Selective modification of bone quality by PTH, pamidronate or raloxifene. J Bone Miner Res 2009 May; 24(5): 800–8
Allen MR. Surface-specific bone formation effects of osteoporosis pharmacological treatments. Clin Rev Bone Miner Metab 2008; 6(1–2): 62–9
Benhamou CL. Effects of osteoporosis medications on bone quality. Joint Bone Spine 2007 Jan; 74(1): 39–47
Meunier PJ, Boivin G, Marie PJ. About the comparison of two anabolic agents, teriparatide and strontium ranelate, in treated osteoporotic women [letter]. J Bone Miner Res 2009 Dec; 24(12): 2066
Rizzoli R, Laroche M, Krieg MA, et al. Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatol Int 2010 Aug; 30(10): 1341–8
Roux S. New treatment targets in osteoporosis. Joint Bone Spine 2010 May; 77(3): 222–8
Acknowledgments
No sources of funding were used in the preparation of this manuscript. J.-Y. Reginster has received consulting fees, payment for serving on advisory boards, lecture fees and/or grant support from Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed, NPS, Theramex, UCB, Merck Sharp and Dohme, Rottapharm, IBSA, Genevrier, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Novo-Nordisk and Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reginster, JY. Antifracture Efficacy of Currently Available Therapies for Postmenopausal Osteoporosis. Drugs 71, 65–78 (2011). https://doi.org/10.2165/11587570-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11587570-000000000-00000