Abstract
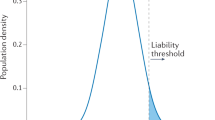
The prevention of coronary heart disease (CHD) is a major public-health goal, but disease architecture is such that a larger proportion of clinical events occur among the average majority than among the high-risk minority—the prevention paradox. Genetic findings over the past few years have resulted in the reopening of the old debate on whether an individualized or a population-based approach to prevention is preferable. Genetic testing is an attractive tool for CHD risk prediction because it is a low-cost, high-fidelity technology with multiplex capability. Moreover, by contrast with nongenetic markers, genotype is invariant and determined from conception, which eliminates biological variability and makes prediction from early life possible. Mindful of the prevention paradox, this Review examines the potential applications and challenges of using genetic information for predicting CHD, focusing on lipid risk factors and drawing on experience in the evaluation of nongenetic risk factors as screening tests for CHD. Many of the issues we discuss hold true for any late-onset common disease with modifiable risk factors and proven preventative strategies.
Key Points
-
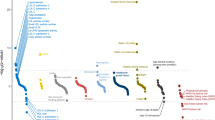
In the past 5 years, the technique of genome-wide association analysis has begun to uncover numerous regions of the genome containing variants that influence blood-lipid levels and risk of coronary heart disease (CHD)
-
Common identified single nucleotide polymorphisms associated with CHD are dispersed across different chromosomes, have modest influence on disease risk, are additive in effect, and explain a small proportion of the observed heritability
-
Challenges in using this type of genetic information to predict individual risk of CHD exist
-
Genome-wide association studies are beginning to provide invaluable new information on causal pathways, which should inform and accelerate the design and development of new treatments
-
Emerging technological advances, including rapid, cost-effective sequencing of whole genomes, are set to further advance understanding of human disease, with prospect of developing better diagnostics and therapies
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Morrison, A. C. et al. Prediction of coronary heart disease risk using a genetic risk score: the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 166, 28–35 (2007).
Paynter, N. P. et al. Cardiovascular disease risk prediction with and without knowledge of genetic variation at chromosome 9p21.3. Ann. Intern. Med. 150, 65–72 (2009).
Kathiresan, S. et al. Polymorphisms associated with cholesterol and risk of cardiovascular events. N. Engl. J. Med. 358, 1240–1249 (2008).
Talmud, P. J. et al. Chromosome 9p21.3 coronary heart disease locus genotype and prospective risk of CHD in healthy middle-aged men. Clin. Chem. 54, 467–474 (2008).
Paynter, N. P. et al. Association between a literature-based genetic risk score and cardiovascular events in women. JAMA 303, 631–637 (2010).
Rosenberg, S. et al. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann. Intern. Med. 153, 425–434 (2010).
Ripatti, S. et al. A multilocus genetic risk score for coronary heart disease: case-control and prospective cohort analyses. Lancet 376, 1393–1400 (2010).
Thaler, R. H. Anomalies. The winner's curse. J. Econ. Perspect. 2, 191–202 (1988).
Qiu, J. & Hayden, E. C. Genomics sizes up. Nature 451, 234 (2008).
Lloyd-Jones, D. et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119, 480–486 (2009).
Lloyd-Jones, D. M., Larson, M. G., Beiser, A. & Levy, D. Lifetime risk of developing coronary heart disease. Lancet 353, 89–92 (1999).
Strong, J. P. et al. Prevalence and extent of atherosclerosis in adolescents and young adults: implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 281, 727–735 (1999).
Ross, R. & Glomset, J. A. The pathogenesis of atherosclerosis. N. Engl. J. Med. 295, 369–377 (1976).
Jones, E. S. & Tobertson, P. W. Coronary artery disease, hypertension, and hypercholesteraemic xanthomatosis. Br. Med. J. 1, 1137 (1948).
Dawber, T. R., Moore, F. E. & Mann, G. V. Coronary heart disease in the Framingham study. Am. J. Public Health Nations Health 47, 4–24 (1957).
Chapman, J. M., Goerke, L. S., Dixon, W., Loveland, D. B. & Phillips, E. Measuring the risk of coronary heart disease in adult population groups. The clinical status of a population group in Los Angeles under observation for two to three years. Am. J. Public Health Nations Health 47, 33–42 (1957).
Dawber, T. R., Meadors, G. F. & Moore, F. E. Jr. Epidemiological approaches to heart disease: the Framingham Study. Am. J. Public Health Nations Health 41, 279–281 (1951).
Doyle, J. T., Heslin, A. S., Hilleboe, H. E., Formel, P. F. & Korns, R. F. A prospective study of degenerative cardiovascular disease in Albany: report of three years' experience. I. Ischemic heart disease. Am. J. Public Health Nations Health 47, 25–32 (1957).
Keys, A. et al. Coronary heart disease among Minnesota business and professional men followed fifteen years. Circulation 28, 381–395 (1963).
Kannel, W. B., Castelli, W. P., Gordon, T. & McNamara, P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann. Intern. Med. 74, 1–12 (1971).
Wilson, P. W. et al. Prediction of coronary heart disease using risk factor categories. Circulation 97, 1837–1847 (1998).
Hopkins, P. N. & Williams, R. R. A survey of 246 suggested coronary risk factors. Atherosclerosis 40, 1–52 (1981).
Collins, R. et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 335, 827–838 (1990).
[No authors listed] Randomised trial of cholesterol lowering in 4,444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383–1389 (1994).
Sacks, F. M. et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 335, 1001–1009 (1996).
Shepherd, J. et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 333, 1301–1307 (1995).
Baigent, C. et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 366, 1267–1278 (2005).
Rose, G. Sick individuals and sick populations. Int. J. Epidemiol. 14, 32–38 (1985).
Law, M. R. & Wald, N. J. Risk factor thresholds: their existence under scrutiny. BMJ 324, 1570–1576 (2002).
Law, M. R., Wald, N. J. & Morris, J. K. The performance of blood pressure and other cardiovascular risk factors as screening tests for ischaemic heart disease and stroke. J. Med. Screen. 11, 3–7 (2004).
Rose, G. Strategy of prevention: lessons from cardiovascular disease. Br. Med. J. (Clin. Res. Ed.) 282, 1847–1851 (1981).
Wald, N. J., Hackshaw, A. K. & Frost, C. D. When can a risk factor be used as a worthwhile screening test? BMJ 319, 1562–1565 (1999).
Law, M. R., Morris, J. K. & Wald, N. J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338, b1665 (2009).
Shah, T. et al. Critical appraisal of CRP measurement for the prediction of coronary heart disease events: new data and systematic review of 31 prospective cohorts. Int. J. Epidemiol. 38, 217–231 (2009).
Hingorani, A. D., Shah, T., Casas, J. P., Humphries, S. E. & Talmud, P. J. C-reactive protein and coronary heart disease: predictive test or therapeutic target? Clin. Chem. 55, 239–255 (2009).
Pietinen, P., Vartiainen, E., Seppanen, R., Aro, A. & Puska, P. Changes in diet in Finland from 1972 to 1992: impact on coronary heart disease risk. Prev. Med. 25, 243–250 (1996).
Pearson, T. A. The epidemiologic basis for population-wide cholesterol reduction in the primary prevention of coronary artery disease. Am. J. Cardiol. 94, 4F–8F (2004).
National Institute for Health and Clinical Excellence. Prevention of cardiovascular disease at the population level: Public health guidance PH25 [online], http://www.nice.org.uk/PH25 (2010).
Olsson, A. G., Mölgaard, J. & von Schenk, H. Synvinolin in hypercholesterolaemia. Lancet 2, 390–391 (1986).
National Institute for Health and Clinical Excellence. Statins for the prevention of cardiovascular events [online], http://www.nice.org.uk/nicemedia/pdf/TA094guidance.pdf (2008).
Graham, I. et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Eur. Heart J. 28, 2375–2414 (2007).
Pearson, T. A. et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation 106, 388–391 (2002).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285, 2486–2497 (2001).
Assmann, G., Cullen, P. & Schulte, H. Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation 105, 310–315 (2002).
Roques, F. et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19,030 patients. Eur. J. Cardiothorac. Surg. 15, 816–822 (1999).
Collins, G. S. & Altman, D. G. An independent external validation and evaluation of QRISK cardiovascular risk prediction: a prospective open cohort study. BMJ 339, b2584 (2009).
Ridker, P. M., Buring, J. E., Rifai, N. & Cook, N. R. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 297, 611–619 (2007).
Wald, N. J., Morris, J. K. & Rish, S. The efficacy of combining several risk factors as a screening test. J. Med. Screen. 12, 197–201 (2005).
Ioannidis, J. P., Ntzani, E. E., Trikalinos, T. A. & Contopoulos-Ioannidis, D. G. Replication validity of genetic association studies. Nat. Genet. 29, 306–309 (2001).
Hirschhorn, J. N., Lohmueller, K., Byrne, E. & Hirschhorn, K. A comprehensive review of genetic association studies. Genet. Med. 4, 45–61 (2002).
Casas, J. P., Cooper, J., Miller, G. J., Hingorani, A. D. & Humphries, S. E. Investigating the genetic determinants of cardiovascular disease using candidate genes and meta-analysis of association studies. Ann. Hum. Genet. 70, 145–169 (2006).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
McPherson, R. et al. A common allele on chromosome 9 associated with coronary heart disease. Science 316, 1488–1491 (2007).
Helgadottir, A. et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 316, 1491–1493 (2007).
Trégouët, D. A. et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat. Genet. 41, 283–285 (2009).
Samani, N. J. et al. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 357, 443–453 (2007).
Kathiresan, S. et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 41, 334–341 (2009).
Erdmann, J. et al. New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat. Genet. 41, 280–282 (2009).
Teslovich, T. M. et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 (2010).
CARDIoGRAM Consortium: Design of the Coronary ARtery DIsease Genome-wide Replication And Meta-Analysis (CARDIoGRAM) Study: A genome-wide association meta-analysis involving more than 22,000 cases and 60,000 controls. Circ. Cardiovasc. Genet. 3, 475–483 (2010).
Waterworth, D. M. et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 30, 2264–2276 (2010).
Samani, N. J. et al. The novel genetic variant predisposing to coronary artery disease in the region of the PSRC1 and CELSR2 genes on chromosome 1 associates with serum cholesterol. J. Mol. Med. 86, 1233–1241 (2008).
Kathiresan, S. et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40, 189–197 (2008).
Holdt, L. M. et al. ANRIL expression is associated with atherosclerosis risk at chromosome 9p21. Arterioscler. Thromb. Vasc. Biol. 30, 620–627 (2010).
Manolio, T. A. Genomewide association studies and assessment of the risk of disease. N. Engl. J. Med. 363, 166–176 (2010).
Talmud, P. J. et al. Gene-centric association signals for lipids and apolipoproteins identified via the HumanCVD BeadChip. Am. J. Hum. Genet. 85, 628–642 (2009).
Manolio, T. A. et al. Finding the missing heritability of complex diseases. Nature 461, 747–753 (2009).
Jakobsdottir, J., Gorin, M. B., Conley, Y. P., Ferrell, R. E. & Weeks, D. E. Interpretation of genetic association studies: markers with replicated highly significant odds ratios may be poor classifiers. PLoS Genet. 5, e1000337 (2009).
Kraft, P. et al. Beyond odds ratios—communicating disease risk based on genetic profiles. Nat. Rev. Genet. 10, 264–269 (2009).
Pepe, M. S., Janes, H., Longton, G., Leisenring, W. & Newcomb, P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am. J. Epidemiol. 159, 882–890 (2004).
Psaty, B. M. et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ. Cardiovasc. Genet. 2, 73–80 (2009).
Ridker, P. M. et al. Rationale, design, and methodology of the Women's Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin. Chem. 54, 249–255 (2008).
Schunkert, H. et al. Repeated replication and a prospective meta-analysis of the association between chromosome 9p21.3 and coronary artery disease. Circulation 117, 1675–1684 (2008).
Chambers, J. C. et al. Common genetic variation near melatonin receptor MTNR1B contributes to raised plasma glucose and increased risk of type 2 diabetes among Indian Asians and European Caucasians. Diabetes 58, 2703–2708 (2009).
Wang, K. et al. Interpretation of association signals and identification of causal variants from genome-wide association studies. Am. J. Hum. Genet. 86, 730–742 (2010).
National Human Genome Research Institute. Human Heredity and Health in Africa Announced in London [online], http://www.genome.gov/27539880 (2010).
International HapMap Project [online], http://www.hapmap.org (2010).
Altshuler, D., Daly, M. J. & Lander, E. S. Genetic mapping in human disease. Science 322, 881–888 (2008).
Clayton, D. G. Prediction and interaction in complex disease genetics: experience in type 1 diabetes. PLoS Genet. 5, e1000540 (2009).
Bodmer, W. & Bonilla, C. Common and rare variants in multifactorial susceptibility to common diseases. Nat. Genet. 40, 695–701 (2008).
Gorlov, I. P., Gorlova, O. Y., Sunyaev, S. R., Spitz, M. R. & Amos, C. I. Shifting paradigm of association studies: value of rare single-nucleotide polymorphisms. Am. J. Hum. Genet. 82, 100–112 (2008).
Marenberg, M. E., Risch, N., Berkman, L. F., Floderus, B. & de Faire, U. Genetic susceptibility to death from coronary heart disease in a study of twins. N. Engl. J. Med. 330, 1041–1046 (1994).
Pharoah, P. D. et al. Polygenic susceptibility to breast cancer and implications for prevention. Nat. Genet. 31, 33–36 (2002).
Pharoah, P. D., Antoniou, A. C., Easton, D. F. & Ponder, B. A. Polygenes, risk prediction, and targeted prevention of breast cancer. N. Engl. J. Med. 358, 2796–2803 (2008).
Wray, N. R., Yang, J., Goddard, M. E. & Visscher, P. M. The genetic interpretation of area under the ROC curve in genomic profiling. PLoS Genet. 6, e1000864 (2010).
Pepe, M. S., Feng, Z. & Gu, J. W. Comments on 'Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond' by M. J. Pencina. et al., Statistics in Medicine (DOI:10.1002/sim.2929). Stat. Med. 27, 173–181 (2008).
Janssens, A. C. et al. The impact of genotype frequencies on the clinical validity of genomic profiling for predicting common chronic diseases. Genet. Med. 9, 528–535 (2007).
Anderson, R. G., Goldstein, J. L. & Brown, M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature 270, 695–699 (1977).
Soria, L. F. et al. Association between a specific apolipoprotein B mutation and familial defective apolipoprotein B-100. Proc. Natl. Acad. Sci. USA 86, 587–591 (1989).
Tybjaerg-Hansen, A., Steffensen, R., Meinertz, H., Schnohr, P. & Nordestgaard, B. G. Association of mutations in the apolipoprotein B gene with hypercholesterolemia and the risk of ischemic heart disease. N. Engl. J. Med. 338, 1577–1584 (1998).
Timms, K. M. et al. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum. Genet. 114, 349–353 (2004).
Patterson, D. & Slack, J. Lipid abnormalities in male and female survivors of myocardial infarction and their first-degree relatives. Lancet 1, 393–399 (1972).
[No authors listed] Mortality in treated heterozygous familial hypercholesterolaemia: implications for clinical management. Scientific Steering Committee on behalf of the Simon Broome Register Group. Atherosclerosis 142, 105–112 (1999).
Neil, A. et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur. Heart J. 29, 2625–2633 (2008).
Kwiterovich, P. O. Jr., Fredrickson, D. S. & Levy, R. I. Familial hypercholesterolemia (one form of familial type II hyperlipoproteinemia). A study of its biochemical, genetic and clinical presentation in childhood. J. Clin. Invest. 53, 1237–1249 (1974).
Leonard, J. V., Whitelaw, A. G., Wolff, O. H., Lloyd, J. K. & Slack, J. Diagnosing familial hypercholesterolaemia in childhood by measuring serum cholesterol. Br. Med. J. 1, 1566–1568 (1977).
Starr, B. et al. Development of sensitive and specific age- and gender-specific low-density lipoprotein cholesterol cutoffs for diagnosis of first-degree relatives with familial hypercholesterolaemia in cascade testing. Clin. Chem. Lab. Med. 46, 791–803 (2008).
Hadfield, S. G. et al. Family tracing to identify patients with familial hypercholesterolaemia: the second audit of the Department of Health Familial Hypercholesterolaemia Cascade Testing Project. Ann. Clin. Biochem. 46, 24–32 (2009).
Taylor, A., Patel, K., Tsedeke, J., Humphries, S. E. & Norbury, G. Mutation screening in patients for familial hypercholesterolaemia (ADH). Clin. Genet. 77, 97–99 (2010).
Wierzbicki, A. S., Humphries, S. E. & Minhas, R. Familial hypercholesterolaemia: summary of NICE guidance. BMJ 337, a1095 (2008).
Umans-Eckenhausen, M. A., Defesche, J. C., Sijbrands, E. J., Scheerder, R. L. & Kastelein, J. J. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 357, 165–168 (2001).
Oliva, J., Lopez-Bastida, J., Moreno, S. G., Mata, P. & Alonso, R. Cost-effectiveness analysis of a genetic screening program in the close relatives of Spanish patients with familial hypercholesterolemia [Spanish]. Rev. Esp. Cardiol. 62, 57–65 (2009).
Pocovi, M., Civeira, F., Alonso, R. & Mata, P. Familial hypercholesterolemia in Spain: case-finding program, clinical and genetic aspects. Semin. Vasc. Med. 4, 67–74 (2004).
Ng, S. B. et al. Targeted capture and massively parallel sequencing of 12 human exomes. Nature 461, 272–276 (2009).
Wald, D. S., Bestwick, J. P. & Wald, N. J. Child-parent screening for familial hypercholesterolaemia: screening strategy based on a meta-analysis. BMJ 335, 599 (2007).
Johansen, C. T. et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 42, 684–687 (2010).
1000 Genomes [online], http://www.1000genomes.org (2010).
Wellcome Trust Sanger Institute. UK10K [online], http://www.uk10k.org (2010).
Genomes Project Consortium. et al. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Caroline, F. W., Philippa, B., Alison, S. & Hilary, B. Realising the benefits of genetics for health. Lancet 376, 1370–1371 (2010).
Structural Genomics Consortium [online], http://www.thesgc.org (2010).
Wolfberg, A. J. Genes on the Web--direct-to-consumer marketing of genetic testing. N. Engl. J. Med. 355, 543–545 (2006).
Genetics and Public Policy Center. Direct-to-consumer genetic testing: empowering or endagering the public? [online], http://www.dnapolicy.org/images/issuebriefpdfs/2006_DTC_Issue_Brief.pdf (2008).
American College of Medicine Genetics Board of Directors. ACMG statement on direct-to-consumer genetic testing. Genet. Med. 6, 60 (2004).
Gollust, S. E., Hull, S. C. & Wilfond, B. S. Limitations of direct-to-consumer advertising for clinical genetic testing. JAMA 288, 1762–1767 (2002).
Hudson, K., Javitt, G., Burke, W. & Byers, P. ASHG Statement* on direct-to-consumer genetic testing in the United States. Obstet. Gynecol. 110, 1392–1395 (2007).
National Human Genome Research Institute. Direct to consumer marketing of genetic tests [online], http://www.genome.gov/12010659 (2004).
Genetic Alliance. Promotion of genetic testing services directly to consumers [online], http://www.geneticalliance.org/ws_display.asp?filter=policy.tmarket (2010).
Shuren, J. Direct-to-consumer genetic testing and the consequences to the public. US Department of Health and Human Services [online], http://www.fda.gov/NewsEvents/Testimony/ucm219925.htm (2010).
Evans, J. P., Dale, D. C. & Fomous, C. Preparing for a consumer-driven genomic age. N. Engl. J. Med. 363, 1099–1103 (2010).
Annes, J. P., Giovanni, M. A. & Murray, M. F. Risks of presymptomatic direct-to-consumer genetic testing. N. Engl. J. Med. 363, 1100–1101 (2010).
Kuehn, B. M. Inconsistent results, inaccurate claims plague direct-to-consumer gene tests. JAMA 301, 1313–1315 (2010).
Humphries, S. E., Yiannakouris, N. & Talmud, P. J. Cardiovascular disease risk prediction using genetic information (gene scores): is it really informative? Curr. Opin. Lipidol. 19, 128–132 (2008).
House of Lords. Science and Technology Committee—Second Report: Genomic Medicine [online], http://www.publications.parliament.uk/pa/ld200809/ldselect/ldsctech/107/10702.htm (2009).
Wald, N. J. & Law, M. R. A strategy to reduce cardiovascular disease by more than 80%. BMJ 326, 1419 (2003).
Yusuf, S. et al. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet 373, 1341–1351 (2009).
Wald, N. J. & Wald, D. S. The polypill concept. Heart 96, 1–4 (2010).
Hlatky, M. A. et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 119, 2408–2416 (2009).
Hindorff, L. A., Junkins, H. A., Hall, P. N., Mehta, J. P. & Manolio, T. A. A Catalog of Published Genome-Wide Association Studies. Available at: http://www.genome.gov/gwastudies. Accessed 22nd October 2010.
Abifadel, M. et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156 (2003).
Willer, C. J. et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40, 161–169 (2008).
Kathiresan, S. et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat. Genet. 41, 56–65 (2009).
Romeo, S. et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119, 70–79 (2009).
Innerarity, T. L. et al. Familial defective apolipoprotein B-100: low density lipoproteins with abnormal receptor binding. Proc. Natl. Acad. Sci. USA 84, 6919–6923 (1987).
Sandhu, M. S. et al. LDL-cholesterol concentrations: a genome-wide association study. Lancet 371, 483–491 (2008).
Sabatti, C. et al. Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat. Genet. 41, 35–46 (2009).
Aulchenko, Y. S. et al. Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat. Genet. 41, 47–55 (2009).
Burkhardt, R. et al. Common SNPs in HMGCR in micronesians and whites associated with LDL-cholesterol levels affect alternative splicing of exon13. Arterioscler. Thromb. Vasc. Biol. 28, 2078–2084 (2008).
Dahlen, G. & Berg, K. Further evidence for the existence of genetically determined metabolic differences between Lp(a+) and Lp(a-) individuals. Clin. Genet. 9, 357–364 (1976).
Wittrup, H. H. et al. A common substitution (Asn291Ser) in lipoprotein lipase is associated with increased risk of ischemic heart disease. J. Clin. Invest. 99, 1606–1613 (1997).
Heid, I. M. et al. Genome-wide association analysis of high-density lipoprotein cholesterol in the population-based KORA study sheds new light on intergenic regions. Circ. Cardiovasc. Genet. 1, 10–20 (2008).
Brooks-Wilson, A. et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22, 336–345 (1999).
Schaeffer, L. et al. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum. Mol. Genet. 15, 1745–1756 (2006).
Karathanasis, S. K., McPherson, J., Zannis, V. I. & Breslow, J. L. Linkage of human apolipoproteins A-I and C-III genes. Nature 304, 371–373 (1983).
Yamakawa-Kobayashi, K. et al. Frequent occurrence of hypoalphalipoproteinemia due to mutant apolipoprotein A-I gene in the population: a population-based survey. Hum. Mol. Genet. 8, 331–336 (1999).
Babaya, N. et al. Association of I27L polymorphism of hepatocyte nuclear factor-1 alpha gene with high-density lipoprotein cholesterol level. J. Clin. Endocrinol. Metab. 88, 2548–2551 (2003).
Fang, D. Z. & Liu, B. W. Polymorphism of HL +1075C, but not -480T, is associated with plasma high density lipoprotein cholesterol and apolipoprotein AI in men of a Chinese population. Atherosclerosis 161, 417–424 (2002).
Kuivenhoven, J. A. et al. The role of a common variant of the cholesteryl ester transfer protein gene in the progression of coronary atherosclerosis. The Regression Growth Evaluation Statin Study Group. N. Engl. J. Med. 338, 86–93 (1998).
Igl, W. et al. Modeling of environmental effects in genome-wide association studies identifies SLC2A2 and HP as novel loci influencing serum cholesterol levels. PLoS Genet. 6, e1000798 (2010).
Ridker, P. M. et al. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: Genomewide analysis among 18,245 initially healthy women from the Women's Genome Health Study. Circ. Cardiovasc. Genet. 2, 26–33 (2009).
Bujo, H. et al. Molecular defect in familial lecithin:cholesterol acyltransferase (LCAT) deficiency: a single nucleotide insertion in LCAT gene causes a complete deficient type of the disease. Biochem. Biophys. Res. Commun. 181, 933–940 (1991).
Yamakawa-Kobayashi, K., Yanagi, H., Endo, K., Arinami, T. & Hamaguchi, H. Relationship between serum HDL-C levels and common genetic variants of the endothelial lipase gene in Japanese school-aged children. Hum. Genet. 113, 311–315 (2003).
Hobbs, H. H., Brown, M. S., Russell, D. W., Davignon, J. & Goldstein, J. L. Deletion in the gene for the low-density-lipoprotein receptor in a majority of French Canadians with familial hypercholesterolemia. N. Engl. J. Med. 317, 734–737 (1987).
Breslow, J. L. et al. Studies of familial type III hyperlipoproteinemia using as a genetic marker the apoE phenotype E2/2. J. Lipid Res. 23, 1224–1235 (1982).
Aouizerat, B. E. et al. Genetic variation of PLTP modulates lipoprotein profiles in hypoalphalipoproteinemia. J. Lipid Res. 47, 787–793 (2006).
Linsel-Nitschke, P. et al. Genetic variation at chromosome 1p13.3 affects sortilin mRNA expression, cellular LDL-uptake and serum LDL levels which translates to the risk of coronary artery disease. Atherosclerosis 208, 183–189 (2010).
Musunuru, K. et al. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 466, 714–719 (2010).
Saxena, R. et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316, 1331–1336 (2007).
O'Doherty, R. M., Lehman, D. L., Telemaque-Potts, S. & Newgard, C. B. Metabolic impact of glucokinase overexpression in liver: lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 48, 2022–2027 (1999).
Slosberg, E. D. et al. Treatment of type 2 diabetes by adenoviral-mediated overexpression of the glucokinase regulatory protein. Diabetes 50, 1813–1820 (2001).
Gretarsdottir, S. et al. Genome-wide association study identifies a sequence variant within the DAB2IP gene conferring susceptibility to abdominal aortic aneurysm. Nat. Genet. 42, 692–697 (2010).
Fujita, P. A. et al. The UCSC Genome Browser database: update 2011. Nucleic Acids Res. doi:10.1093/nar/gkq963.
Acknowledgements
A. D. Hingorani and S. E. Humphries contributed equally to this article. M. V. Holmes is funded by a Population Health Scientist Fellowship from the Medical Research Council (G0802432). The British Heart Foundation supports P. J Talmud (RG/08/008), A. D. Hingorani (FS 05/125), and S. E Humphries (PG/07/133/24,260). This work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centers funding scheme.
Author information
Authors and Affiliations
Contributions
All authors contributed to discussion of content for the article, researched data to include in the manuscript, reviewed and edited the manuscript before submission, and revised the manuscript in response to the peer-reviewers' comments. M. V. Holmes, S. Harrison, A. D. Hingorani and S. E Humphries were also involved in writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
A. Hingorani is a member of the Editorial Board of the Drug and Therapeutics Bulletin (International Society of Drug Bulletins). He has acted as a consultant to London Genetics and to GlaxoSmithKline. He has received honoraria for speaking at educational meetings but has donated these in large part to various medical charities. The other authors declare no competing interests.
Supplementary information
Supplementary Table 1
Loci and genes for lipids, HDL-C, LDL-C and cholesterol identified by genome wide association studies to date (DOC 533 kb)
Supplementary Table 2
Loci and genes for myocardial infarction and/or coronary artery disease identified by genome wide association studies to date (DOC 79 kb)
Supplementary Box 1
Tools for assessing predictive utility of gene markers (DOC 40 kb)
Supplementary Information
Derivation of values for Table 7 (DOC 43 kb)
Rights and permissions
About this article
Cite this article
Holmes, M., Harrison, S., Talmud, P. et al. Utility of genetic determinants of lipids and cardiovascular events in assessing risk. Nat Rev Cardiol 8, 207–221 (2011). https://doi.org/10.1038/nrcardio.2011.6
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2011.6
This article is cited by
-
Integrating genomics with biomarkers and therapeutic targets to invigorate cardiovascular drug development
Nature Reviews Cardiology (2021)
-
Genetic Risk Factors and Mendelian Randomization in Cardiovascular Disease
Current Cardiology Reports (2015)
-
Wrapper-based selection of genetic features in genome-wide association studies through fast matrix operations
Algorithms for Molecular Biology (2012)
-
Mitochondrial DNA 5178 C/A polymorphism influences the effects of habitual smoking on the risk of dyslipidemia in middle-aged Japanese men
Lipids in Health and Disease (2012)
-
Initiation of Statin Therapy: Are There Age Limits?
Current Atherosclerosis Reports (2012)