Abstract
Background
The effects of disclosing financial interests to potential research participants are not well understood.
Objective
To examine the effects of financial interest disclosures on potential research participants’ attitudes toward clinical research.
Design and Participants
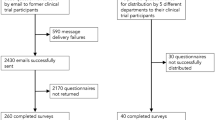
Computerized experiment conducted with 3,623 adults in the United States with either diabetes mellitus or asthma, grouped by lesser and greater severity. Respondents read a description of a hypothetical clinical trial relevant to their diagnosis that included a financial disclosure statement. Respondents received 1 of 5 disclosure statements.
Measurements
Willingness to participate in the hypothetical clinical trial, relative importance of information about the financial interest, change in trust after reading the disclosure statement, surprise regarding the financial interest, and perceived effect of the financial interest on the quality of the clinical trial.
Results
Willingness to participate in the hypothetical clinical trial did not differ substantially among the types of financial disclosures. Respondents viewed the disclosed information as less important than other factors in deciding to participate. Disclosures were associated with some respondents trusting the researchers less, although trust among some respondents increased. Most respondents were not surprised to learn of financial interests. Researchers owning equity were viewed as more troubling than researchers who were compensated for the costs of research through per capita payments.
Conclusions
Aside from a researcher holding an equity interest, the disclosure to potential research participants of financial interests in research, as recommended in recent policies, is unlikely to affect willingness to participate in research.
Similar content being viewed by others
References
Blumenthal D. Academic–industrial relationships in the life sciences. N Engl J Med. 2003;349:2452–9.
Bodenheimer T. Uneasy alliance—clinical investigators and the pharmaceutical industry. N Engl J Med. 2000;342:1539–44.
Korenman SG. Conflicts of interest and commercialization of research. Acad Med. 1993;68(9 Suppl):S18–22.
Shalala D. Protecting research subjects—what must be done. N Engl J Med. 2000;343:808–10.
Thompson DF. Understanding financial conflicts of interest. N Engl J Med. 1993;329:573–6.
National Bioethics Advisory Commission. Ethical and policy issues in research involving human participants, Vol. 1. Bethesda, Md: National Bioethics Advisory Commission; August 2001.
Department of Health and Human Services. Financial relationships and interests in research involving human subjects: guidance for human subject protection. Fed Regist. 2004;69:26393–7.
Kim SY, Millard RW, Nisbet P, Cox C, Caine ED. Potential research participants’ views regarding researcher and institutional conflicts of interest. J Med Ethics. 2004;30:73–9.
Hampson LA, Agrawal M, Joffe S, Gross CP, Verter J, Emanuel EJ. Patients’ views on financial conflicts of interest in cancer research trials. N Engl J Med. 2006;355:2330–7.
Weinfurt KP, Friedman JY, Allsbrook JS, Dinan MA, Hall M, Sugarman J. Views of potential research participants on financial conflicts of interest: barriers and opportunities for effective disclosure. J Gen Intern Med. 2006;21:901–6.
Grady C, Horstmann E, Sussman JS, Hull SC. The limits of disclosure: what research subjects want to know about investigator financial interests. J Law Med Ethics. 2006;34:592–9.
Weinfurt KP, Dinan MA, Allsbrook JS, et al. Policies of academic medical centers for disclosing conflicts of interest to potential research participants. Acad Med. 2006;81:113–8.
Weinfurt KP, Allsbrook JS, Friedman JY, et al. Developing model language for disclosing financial interests to potential clinical research participants. IRB Advis. 2007;29:1–5.
Hall MA, Camacho F, Lawlor JS, DePuy V, Sugarman J, Weinfurt KP. Measuring trust in medical researchers. Med Care. 2006;44:1048–53.
Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
Weinfurt KP, Friedman JY, Dinan MA, et al. Disclosing conflicts of interest in clinical research: views of institutional review boards, conflict of interest committees, and investigators. J Law Med Ethics. 2006;34:581–91.
Hall MA, Kidd KE, Dugan E. Disclosure of physician incentives: do practices satisfy purposes? Health Aff (Millwood). 2000;19:156–64.
Kass NE, Sugarman J, Faden R, Schoch-Spana M. Trust, the fragile foundation of contemporary biomedical research. Hastings Cent Rep. 1996;26:25–9.
New survey shows public perception of opportunity to participate in clinical trials has decreased slightly from last year. Harris Interactive Healthcare News. 2005;5(6):1–14. Available at: http://www.harrisinteractive.com/news/allnewsbydate.asp?NewsID=941. Accessed February 9, 2007.
Task Force on Financial Conflicts of Interest in Clinical Research. Protecting Subjects, Preserving Trust, Promoting Progress—Policy and Guidelines for the Oversight of Individual Financial Interests in Human Subjects Research. Washington, DC: Association of American Medical Colleges; 2001:1–23.
Acknowledgments
We thank Scott Y. Kim of the University of Michigan for commenting on an early draft of the manuscript; Diana B. McNeill, Mark N. Feinglos, and Peter S. Kussin of Duke University, and Peter B. Terry, Frederick L. Brancati, and Jerry A. Krishnan of The Johns Hopkins University for assistance with developing items with which to categorize disease severity among survey respondents; and Damon Seils of Duke University for editorial assistance and manuscript preparation.
Funding/support
This study was supported by grant R01HL075538-01 from the US National Heart, Lung, and Blood Institute to Dr Sugarman. This work was independent of the funding source. The sponsor had no involvement in the study design; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication.
Conflicts of interest
None disclosed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 44.0 kb)
Rights and permissions
About this article
Cite this article
Weinfurt, K.P., Hall, M.A., Dinan, M.A. et al. Effects of Disclosing Financial Interests on Attitudes Toward Clinical Research. J GEN INTERN MED 23, 860–866 (2008). https://doi.org/10.1007/s11606-008-0590-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-008-0590-4