Abstract
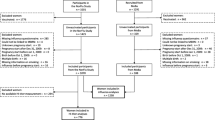
Although vaccines against influenza can reduce maternal morbidity and mortality, large-scale data on adverse effects in the offspring are scarce. Historical cohort study in Stockholm County, Sweden. We linked H1N1 vaccination data (Pandemrix®, a mono-valent AS03 adjuvanted H1N1 vaccine) with pregnancy and birth data from 21,087 women with singleton offspring conceived between February 2009 and January 2010 (vaccinated during pregnancy: n = 13,297 vs. unvaccinated: n = 7,790). Data were analysed by conceptualizing the observational cohort as a series of nested cohorts defined at each week of gestation. Logistic regression estimated odds ratios (ORs) for low birth weight (LBW, <2,500 g), preterm birth (<37 completed weeks), small-for-gestational age (SGA, <10th percentile of the gestational age-specific birth weight within the cohort), low 5-min Apgar score (<7), and caesarean section. Data were adjusted for potential confounders, including maternal age, body mass index, smoking, parity, civil status and comorbidities. Compared with infants of non-vaccinated women, infants of vaccinated women had similar adjusted ORs (95 % CI) for LBW (0.91; 0.79–1.04), preterm birth (0.99; 0.89–1.10), SGA (0.97; 0.90–1.05), low Apgar score (1.05, 0.84–1.31), and a marginal risk reduction for caesarean section (0.94, 0.89–0.99). H1N1 vaccination during pregnancy, using an AS03-adjuvanted vaccine, does not appear to adversely influence offspring risks of LBW, preterm birth, SGA, or low Apgar score. Our results suggest that this vaccine is safe for the offspring when used in different stages of pregnancy.


Similar content being viewed by others
Abbreviations
- CI:
-
Confidence interval
- LBW:
-
Low birth weight
- OR:
-
Odds ratio
- SGA:
-
Small for gestational age
References
Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–40.
Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–86.
Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–15.
Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201.
Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Alpuche-Aranda CM, Chapela IB, Zavala EP, Guevara DM, Checchi F, Garcia E, Hugonnet S, Roth C. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324(5934):1557–61.
Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–44.
WHO. New influenza A (H1N1) virus: WHO guidance on public health measures, 11 June 2009. Wkly Epidemiol Rec. 2009;84(26):261–4.
Johansen K, Nicoll A, Ciancio BC, Kramarz P. Pandemic influenza A(H1N1) 2009 vaccines in the European Union. Euro Surveill. 2009;14(41):19361.
Cox S, Posner SF, McPheeters M, Jamieson DJ, Kourtis AP, Meikle S. Hospitalizations with respiratory illness among pregnant women during influenza season. Obstet Gynecol. 2006;107(6):1315–22.
Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14(1):95–100.
Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–8.
Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27–35.
Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, Chu SY, Sackoff JE, Jamieson DJ, Fine AD, Shapiro-Mendoza CK, Jones LE, Uyeki TM, Balter S, Bish CL, Finelli L, Honein MA. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115(4):717–26.
Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Tabony L, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–25.
Läkemedelsverkets nytta/riskvärdering av vaccination av gravida med Pandemrix [Benefits and risks of vaccinations with Pandemrix during pregnancy. Recommendations by the Swedish Medical Products Agency]. Uppsala: Swedish Medical Products Agency, 2009.
Brent RL. Risks and benefits of immunizing pregnant women: the risk of doing nothing. Reprod Toxicol. 2006;21(4):383–9.
Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, Barash F, Arana J, Brantley MD, Ding H, Singleton JA, Walton K, Haber P, Lewis P, Yue X, Destefano F, Vellozzi C. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the vaccine adverse event reporting system. Am J Obstet Gynecol. 2011;205(5):473e1–9.
Huang WT, Chen WC, Teng HJ, Huang WI, Huang YW, Hsu CW, Chuang JH. Adverse events following pandemic A (H1N1) 2009 monovalent vaccines in pregnant women—Taiwan, November 2009-August 2010. PLoS ONE. 2011;6(8):e23049.
Kallen B, Olausson P. Vaccination against H1N1 influenza with Pandemrix((R)) during pregnancy and delivery outcome: a Swedish register study. BJOG. 2012;119(13):1583–90.
Fell DB, Sprague AE, Liu N, Yasseen AS 3rd, Wen SW, Smith G, Walker MC. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102(6):e33–40.
Pasternak B, Svanstrom H, Molgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, Hviid A. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA. 2012;308(2):165–74.
Pasternak B, Svanstrom H, Molgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, Hviid A. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ. 2012;344:e2794.
Dodds L, Macdonald N, Scott J, Spencer A, Allen VM, McNeil S. The association between influenza vaccine in pregnancy and adverse neonatal outcomes. J Obstet Gynaecol Can. 2012;34(8):714–20.
Heikkinen T, Young J, van Beek E, Franke H, Verstraeten T, Weil JG, Della Cioppa G. Safety of MF59-adjuvanted A/H1N1 influenza vaccine in pregnancy: a comparative cohort study. Am J Obstet Gynecol. 2012;207(3):177e1–8.
Lin TH, Lin SY, Lin CH, Lin RI, Lin HC, Chiu TH, Cheng PJ, Lee CN. AdimFlu-S((R)) influenza A (H1N1) vaccine during pregnancy: the Taiwanese Pharmacovigilance Survey. Vaccine. 2012;30(16):2671–5.
Tavares F, Nazareth I, Monegal JS, Kolte I, Verstraeten T, Bauchau V. Pregnancy and safety outcomes in women vaccinated with an AS03-adjuvanted split virion H1N1 (2009) pandemic influenza vaccine during pregnancy: a prospective cohort study. Vaccine. 2011;29(37):6358–65.
Omon E, Damase-Michel C, Hurault-Delarue C, Lacroix I, Montastruc JL, Oustric S, Escourrou B. Non-adjuvanted 2009 influenza A (H1N1)v vaccine in pregnant women: the results of a French prospective descriptive study. Vaccine. 2011;29(52):9649–54.
Mackenzie IS, MacDonald TM, Shakir S, Dryburgh M, Mantay BJ, McDonnell P, Layton D. Influenza H1N1 (swine flu) vaccination: a safety surveillance feasibility study using self-reporting of serious adverse events and pregnancy outcomes. Br J Clin Pharmacol. 2012;73(5):801–11.
Oppermann M, Fritzsche J, Weber-Schoendorfer C, Keller-Stanislawski B, Allignol A, Meister R, Schaefer C. A(H1N1)v2009: a controlled observational prospective cohort study on vaccine safety in pregnancy. Vaccine. 2012;30(30):4445–52.
Rubinstein F, Micone P, Bonotti A, Wainer V, Schwarcz A, Augustovski F, Pichon Riviere A, Karolinski A. Influenza A/H1N1 MF59 adjuvanted vaccine in pregnant women and adverse perinatal outcomes: multicentre study. BMJ. 2013;346:f393.
Richards JL, Hansen C, Bredfeldt C, Bednarczyk RA, Steinhoff MC, Adjaye-Gbewonyo D, Ault K, Gallagher M, Orenstein W, Davis RL, Omer SB. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis. 2013;56(9):1216–22.
Haberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, Skrondal A, Cappelen I, Engeland A, Aavitsland P, Madsen S, Buajordet I, Furu K, Nafstad P, Vollset SE, Feiring B, Nokleby H, Magnus P, Stoltenberg C. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368(4):333–40.
Bardage C, Persson I, Ortqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956.
Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsen P, Saarenpaa-Heikkila O, Kilpi T. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS ONE. 2012;7(3):e33536.
O’Flanagan D, Bonner C, Crowe C, Lynch B, Sweeney B, Gilvarry J, Johnsson H, Cotter S, Barret A-S. Investigation of an increase in the incidence of narcolepsy in children and adolescents in 2009 and 2010. Final report of national narcolepsy study steering committee, 2012.
Hernan MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, Manson JE, Robins JM. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–79.
Ortqvist A, Berggren I, Insulander M, de Jong B, Svenungsson B. Effectiveness of an adjuvanted monovalent vaccine against the 2009 pandemic strain of influenza A(H1N1)v, in Stockholm County, Sweden. Clin Infect Dis. 2011;52(10):1203–11.
Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–67.
Szegö J. Bebyggelsens mosaik [The mosaic of the settlement]. Stockholm, 2009.
Hogberg U, Larsson N. Early dating by ultrasound and perinatal outcome. A cohort study. Acta Obstet Gynecol Scand. 1997;76(10):907–12.
Ortqvist A, Granath F, Askling J, Hedlund J. Influenza vaccination and mortality: prospective cohort study of the elderly in a large geographical area. Eur Respir J. 2007;30(3):414–22.
Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy. 2004;24(6):743–9.
Statistikdatabasen [Statistics Database]. Stockholm: National Board of Health and Welfare, 2012.
Acknowledgments
This project was supported by grants from the Swedish Research Council (Medicine), and the Swedish Council for Working Life and Social Research (FAS).
Ethical approval
This project (2009/1952-31/4) was approved by the Research Ethics Committee of the Karolinska Institute, Sweden on January 13, 2010.
Conflict of interest
JFL was funded by the Swedish Research Council (Medicine), OS was funded by the Swedish Society of Medicine. LR was partially supported by grants from the Compagnia san Paolo/Firms and the Italian Association for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclaimer This manuscript represents the views of the authors, not necessarily those of the Swedish drug regulatory agency (Medical Products Agency) where one of the authors is employed (IP).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ludvigsson, J.F., Zugna, D., Cnattingius, S. et al. Influenza H1N1 vaccination and adverse pregnancy outcome. Eur J Epidemiol 28, 579–588 (2013). https://doi.org/10.1007/s10654-013-9813-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-013-9813-z