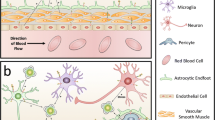
Abstract
Vascular dysfunction has a critical role in Alzheimer’s disease (AD). Recent data from brain imaging studies in humans and animal models suggest that cerebrovascular dysfunction may precede cognitive decline and onset of neurodegenerative changes in AD and AD models. Cerebral hypoperfusion and impaired amyloid β-peptide (Aβ) clearance across the blood–brain barrier (BBB) may contribute to the onset and progression of dementia AD type. Decreased cerebral blood flow (CBF) negatively affects the synthesis of proteins required for memory and learning, and may eventually lead to neuritic injury and neuronal death. Impaired clearance of Aβ from the brain by the cells of the neurovascular unit may lead to its accumulation on blood vessels and in brain parenchyma. The accumulation of Aβ on the cerebral blood vessels, known as cerebral amyloid angiopathy (CAA), is associated with cognitive decline and is one of the hallmarks of AD pathology. CAA can severely disrupt the integrity of the blood vessel wall resulting in micro or macro intracerebral bleedings that exacerbates neurodegenerative process and inflammatory response and may lead to hemorrhagic stroke, respectively. Here, we review the role of the neurovascular unit and molecular mechanisms in vascular cells behind AD and CAA pathogenesis. First, we discuss apparent vascular changes, including the cerebral hypoperfusion and vascular degeneration that contribute to different stages of the disease process in AD individuals. We next discuss the role of the low-density lipoprotein receptor related protein-1 (LRP), a key Aβ clearance receptor at the BBB and along the cerebrovascular system, whose expression is suppressed early in AD. We also discuss how brain-derived apolipoprotein E isoforms may influence Aβ clearance across the BBB. We then review the role of two interacting transcription factors, myocardin and serum response factor, in cerebral vascular cells in controlling CBF responses and LRP-mediated Aβ clearance. Finally, we discuss the role of microglia and perivascular macrophages in Aβ clearance from the brain. The data reviewed here support an essential role of neurovascular and BBB mechanisms in contributing to both, onset and progression of AD.



Similar content being viewed by others
References
Alonzo NC, Hyman BT, Rebeck GW, Greenberg SM (1998) Progression of cerebral amyloid angiopathy: accumulation of amyloid-beta40 in affected vessels. J Neuropathol Exp Neurol 57:353–359. doi:10.1097/00005072-199804000-00008
Asahina M, Yoshiyama Y, Hattori T (2001) Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer’s disease brain. Clin Neuropathol 20:60–63
Attems J, Jellinger KA, Lintner F (2005) Alzheimer’s disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol 110:222–231. doi:10.1007/s00401-005-1064-y
Attems J, Quass M, Jellinger KA, Lintner F (2007) Topographical distribution of cerebral amyloid angiopathy and its effect on cognitive decline are influenced by Alzheimer disease pathology. J Neurol Sci 257:49–55. doi:10.1016/j.jns.2007.01.013
Bailey TL, Rivara CB, Rocher AB, Hof PR (2004) The nature and effects of cortical microvascular pathology in aging and Alzheimer’s disease. Neurol Res 26:573–578. doi:10.1179/016164104225016272
Beach TG, Wilson JR, Sue LI et al (2007) Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol 113:13–21. doi:10.1007/s00401-006-0136-y
Begley DJ, Brightman MW (2003) Structural and functional aspects of the blood–brain barrier. Prog Drug Res 61:39–78
Bell RD, Deane R, Chow N et al (2009) SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol 11:143–153. doi:10.1038/ncb1819
Bell RD, Sagare AP, Friedman AE et al (2007) Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27:909–918
Bertram L, Tanzi RE (2008) Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 9:768–778. doi:10.1038/nrn2494
Berzin TM, Zipser BD, Rafii MS et al (2000) Agrin and microvascular damage in Alzheimer’s disease. Neurobiol Aging 21:349–355. doi:10.1016/S0197-4580(00)00121-4
Bradbury M (1979) Cerebral arterial supply. In: Bradbury M (ed) The concept of a blood–brain barrier. Wiley, New York, pp 18–19
Chen J, Kitchen CM, Streb JW, Miano JM (2002) Myocardin: a component of a molecular switch for smooth muscle differentiation. J Mol Cell Cardiol 34:1345–1356. doi:10.1006/jmcc.2002.2086
Cheng T, Petraglia AL, Li Z et al (2006) Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med 12:1278–1285. doi:10.1038/nm1498
Chow N, Bell RD, Deane R et al (2007) Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci USA 104:823–828. doi:10.1073/pnas.0608251104
Clifford PM, Zarrabi S, Siu G et al (2007) Abeta peptides can enter the brain through a defective blood–brain barrier and bind selectively to neurons. Brain Res 1142:223–236. doi:10.1016/j.brainres.2007.01.070
Corder EH, Saunders AM, Risch NJ et al (1994) Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 7:180–184. doi:10.1038/ng0694-180
Corder EH, Saunders AM, Strittmatter WJ et al (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–923. doi:10.1126/science.8346443
Davis J, Xu F, Deane R et al (2004) Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. J Biol Chem 279:20296–20306. doi:10.1074/jbc.M312946200
Davson H, Welch K, Segal MB (1987) Morphological aspects of the barriers: perivascular spaces. In: Davson H (ed) The physiology and pathophysiology of the cerebrospinal fluid. Churchill Livingstone, New York, pp 135–137
de la Torre JC (2004) Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 3:184–190. doi:10.1016/S1474-4422(04)00683-0
Deane R, Bell RD, Sagare A, Zlokovic BZ (2009) Clearance of amyloid-beta peptide across the blood–brain barrier: implications for therapies in Alzhiemer’s disease. CNS Neurol Disord Drug Targets 8. doi:10.2174/187152709787601867 (in press)
Deane R, Du Yan S, Submamaryan RK et al (2003) RAGE mediates amyloid-beta peptide transport across the blood–brain barrier and accumulation in brain. Nat Med 9:907–913. doi:10.1038/nm890
Deane R, Sagare A, Hamm K et al (2008) apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 118:4002–4013. doi:10.1172/JCI36663
Deane R, Wu Z, Sagare A et al (2004) LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron 43:333–344. doi:10.1016/j.neuron.2004.07.017
Drzezga A, Lautenschlager N, Siebner H et al (2003) Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging 30:1104–1113. doi:10.1007/s00259-003-1194-1
El Khoury J, Toft M, Hickman SE et al (2007) Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13:432–438. doi:10.1038/nm1555
Ervin JF, Pannell C, Szymanski M, Welsh-Bohmer K, Schmechel DE, Hulette CM (2004) Vascular smooth muscle actin is reduced in Alzheimer disease brain: a quantitative analysis. J Neuropathol Exp Neurol 63:735–741
Farkas E, Luiten PG (2001) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol 64:575–611. doi:10.1016/S0301-0082(00)00068-X
Fiala M, Lin J, Ringman J et al (2005) Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 7:221–232 discussion 55–62
Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL Jr, del Zoppo GJ (2004) Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke 35:998–1004. doi:10.1161/01.STR.0000119383.76447.05
Giri R, Shen Y, Stins M et al (2000) Beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol 279:C1772–C1781
Girouard H, Iadecola C (2006) Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100:328–335. doi:10.1152/japplphysiol.00966.2005
Grabowski TJ, Cho HS, Vonsattel JP, Rebeck GW, Greenberg SM (2001) Novel amyloid precursor protein mutation in an Iowa family with dementia and severe cerebral amyloid angiopathy. Ann Neurol 49:697–705. doi:10.1002/ana.1009
Grammas P, Yamada M, Zlokovic B (2002) The cerebromicrovasculature: a key player in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 4:217–223
Greenberg SM, Gurol ME, Rosand J, Smith EE (2004) Amyloid angiopathy-related vascular cognitive impairment. Stroke 35:2616–2619. doi:10.1161/01.STR.0000143224.36527.44
Griffin JH, Zlokovic B, Fernandez JA (2002) Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol 39:197–205. doi:10.1053/shem.2002.34093
Hawkes CA, McLaurin J (2009) Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci USA 106:1261–1266. doi:10.1073/pnas.0805453106
Hendriks L, van Duijn CM, Cras P et al (1992) Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the beta-amyloid precursor protein gene. Nat Genet 1:218–221. doi:10.1038/ng0692-218
Hickman SE, Allison EK, El Khoury J (2008) Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci 28:8354–8360. doi:10.1523/JNEUROSCI.0616-08.2008
Hirao K, Ohnishi T, Hirata Y et al (2005) The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage 28:1014–1021. doi:10.1016/j.neuroimage.2005.06.066
Hofman A, Ott A, Breteler MM et al (1997) Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet 349:151–154. doi:10.1016/S0140-6736(96)09328-2
Honig LS, Kukull W, Mayeux R (2005) Atherosclerosis and AD: analysis of data from the US National Alzheimer’s Coordinating Center. Neurology 64:494–500
Hunt A, Schonknecht P, Henze M, Seidl U, Haberkorn U, Schroder J (2007) Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease. Psychiatry Res 155:147–154. doi:10.1016/j.pscychresns.2006.12.003
Iadecola C (2004) Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5:347–360. doi:10.1038/nrn1387
Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T (1993) Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci 116:135–141. doi:10.1016/0022-510X(93)90317-R
Jellinger KA (2002) Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm 109:813–836. doi:10.1007/s007020200068
Johnson NA, Jahng GH, Weiner MW et al (2005) Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 234:851–859. doi:10.1148/radiol.2343040197
Jung SS, Zhang W, Van Nostrand WE (2003) Pathogenic A beta induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J Neurochem 85:1208–1215. doi:10.1046/j.1471-4159.2003.01745.x
Kalaria RN, Hedera P (1995) Differential degeneration of the cerebral microvasculature in Alzheimer’s disease. Neuroreport 6:477–480. doi:10.1097/00001756-199502000-00018
Kalaria RN, Pax AB (1995) Increased collagen content of cerebral microvessels in Alzheimer’s disease. Brain Res 705:349–352. doi:10.1016/0006-8993(95)01250-8
Kalback W, Esh C, Castano EM et al (2004) Atherosclerosis, vascular amyloidosis and brain hypoperfusion in the pathogenesis of sporadic Alzheimer’s disease. Neurol Res 26:525–539. doi:10.1179/016164104225017668
Kawai M, Kalaria RN, Cras P et al (1993) Degeneration of vascular muscle cells in cerebral amyloid angiopathy of Alzheimer disease. Brain Res 623:142–146. doi:10.1016/0006-8993(93)90021-E
Kida S, Steart PV, Zhang ET, Weller RO (1993) Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol 85:646–652. doi:10.1007/BF00334675
Kim HC, Yamada K, Nitta A et al (2003) Immunocytochemical evidence that amyloid beta (1–42) impairs endogenous antioxidant systems in vivo. Neuroscience 119:399–419. doi:10.1016/S0306-4522(02)00993-4
Li S, Wang DZ, Wang Z, Richardson JA, Olson EN (2003) The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci USA 100:9366–9370. doi:10.1073/pnas.1233635100
Llorente-Cortes V, Costales P, Bernues J, Camino-Lopez S, Badimon L (2006) Sterol regulatory element-binding protein-2 negatively regulates low density lipoprotein receptor-related protein transcription. J Mol Biol 359:950–960. doi:10.1016/j.jmb.2006.04.008
Martel CL, Mackic JB, Matsubara E et al (1997) Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood–brain barrier transport of circulating Alzheimer’s amyloid beta. J Neurochem 69:1995–2004
McComb JG, Zlokovic BV (1994) Cerebrospinal fluid and the blood–brain interface. In: Cheek WR (ed) Pediatric neurosurgery: surgery of the developing nervous system, 3rd edn. Saunders, Philladelphia, pp 180–198
Monro OR, Mackic JB, Yamada S et al (2002) Substitution at codon 22 reduces clearance of Alzheimer’s amyloid-beta peptide from the cerebrospinal fluid and prevents its transport from the central nervous system into blood. Neurobiol Aging 23:405–412. doi:10.1016/S0197-4580(01)00317-7
Mosconi L, Sorbi S, de Leon MJ et al (2006) Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J Nucl Med 47:1778–1786
Nilsberth C, Westlind-Danielsson A, Eckman CB et al (2001) The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci 4:887–893. doi:10.1038/nn0901-887
Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C (2004) Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab 24:334–342. doi:10.1097/01.WCB.0000105800.49957.1E
Parks JK, Smith TS, Trimmer PA, Bennett JP Jr, Parker WD Jr (2001) Neurotoxic Abeta peptides increase oxidative stress in vivo through NMDA-receptor and nitric-oxide-synthase mechanisms, and inhibit complex IV activity and induce a mitochondrial permeability transition in vitro. J Neurochem 76:1050–1056. doi:10.1046/j.1471-4159.2001.00112.x
Peppiatt CM, Howarth C, Mobbs P, Attwell D (2006) Bidirectional control of CNS capillary diameter by pericytes. Nature 443:700–704. doi:10.1038/nature05193
Pettersen JA, Sathiyamoorthy G, Gao FQ et al (2008) Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol 65:790–795. doi:10.1001/archneur.65.6.790
Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO (2003) Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol 29:106–117. doi:10.1046/j.1365-2990.2003.00424.x
Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA (1997) Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem 272:23946–23951. doi:10.1074/jbc.272.38.23946
Reynolds PR, Mucenski ML, Le Cras TD, Nichols WC, Whitsett JA (2004) Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J Biol Chem 279:37124–37132. doi:10.1074/jbc.M405254200
Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P (2005) Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. Neuroimage 26:1078–1085. doi:10.1016/j.neuroimage.2005.03.022
Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8:205–216. doi:10.1016/S1474-4422(09)70016-X
Ruitenberg A, den Heijer T, Bakker SL et al (2005) Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol 57:789–794. doi:10.1002/ana.20493
Sagare A, Deane R, Bell RD et al (2007) Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med 13:1029–1031. doi:10.1038/nm1635
Saido TC, Iwata N (2006) Metabolism of amyloid beta peptide and pathogenesis of Alzheimer’s disease. Towards presymptomatic diagnosis, prevention and therapy. Neurosci Res 54:235–253. doi:10.1016/j.neures.2005.12.015
Samuraki M, Matsunari I, Chen WP et al (2007) Partial volume effect-corrected FDG PET and grey matter volume loss in patients with mild Alzheimer’s disease. Eur J Nucl Med Mol Imaging 34:1658–1669. doi:10.1007/s00259-007-0454-x
Santpere G, Puig B, Ferrer I (2007) Oxidative damage of 14-3-3 zeta and gamma isoforms in Alzheimer’s disease and cerebral amyloid angiopathy. Neuroscience 146:1640–1651. doi:10.1016/j.neuroscience.2007.03.013
Scheibel AB, Duong TH, Jacobs R (1989) Alzheimer’s disease as a capillary dementia. Ann Med 21:103–107. doi:10.3109/07853898909149194
Segal MB, Preston JE, Collis CS, Zlokovic BV (1990) Kinetics and Na independence of amino acid uptake by blood side of perfused sheep choroid plexus. Am J Physiol 258:F1288–F1294
Shankar GM, Li S, Mehta TH et al (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14:837–842. doi:10.1038/nm1782
Shibata M, Yamada S, Kumar SR et al (2000) Clearance of Alzheimer’s amyloid-beta (1-40) peptide from brain by LDL receptor-related protein-1 at the blood–brain barrier. J Clin Invest 106:1489–1499. doi:10.1172/JCI10498
Sun Q, Chen G, Streb JW et al (2006) Defining the mammalian CArGome. Genome Res 16:197–207. doi:10.1101/gr.4108706
Tagliavini FRG, Padovani A, Magoni M, Andora G, Sgarzi M, Bizzi ASM, Carella F, Morbin M, Giaccone G, Bugiani O (1999) A new beta APP mutation related to hereditary cerebral haemorrhage. Alzheimers Rep 2:S28
Tamaki C, Ohtsuki S, Iwatsubo T et al (2006) Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid beta-peptide by the liver. Pharm Res 23:1407–1416. doi:10.1007/s11095-006-0208-7
Tanzi RE, Bertram L (2005) Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell 120:545–555. doi:10.1016/j.cell.2005.02.008
Thal DR, Griffin WS, de Vos RA, Ghebremedhin E (2008) Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol 115:599–609. doi:10.1007/s00401-008-0366-2
Trembath D, Ervin JF, Broom L et al (2007) The distribution of cerebrovascular amyloid in Alzheimer’s disease varies with ApoE genotype. Acta Neuropathol 113:23–31. doi:10.1007/s00401-006-0162-9
Urmoneit B, Prikulis I, Wihl G et al (1997) Cerebrovascular smooth muscle cells internalize Alzheimer amyloid beta protein via a lipoprotein pathway: implications for cerebral amyloid angiopathy. Lab Invest 77:157–166
Van Broeckhoven C, Haan J, Bakker E et al (1990) Amyloid beta protein precursor gene and hereditary cerebral hemorrhage with amyloidosis (Dutch). Science 248:1120–1122. doi:10.1126/science.1971458
van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM (2007) Atherosclerosis and risk for dementia. Ann Neurol 61:403–410. doi:10.1002/ana.21073
Verbeek MM, Otte-Holler I, van den Born J et al (1999) Agrin is a major heparan sulfate proteoglycan accumulating in Alzheimer’s disease brain. Am J Pathol 155:2115–2125
Verbeek MM, Van Nostrand WE, Otte-Holler I, Wesseling P, De Waal RM (2000) Amyloid-beta-induced degeneration of human brain pericytes is dependent on the apolipoprotein E genotype. Ann N Y Acad Sci 903:187–199. doi:10.1111/j.1749-6632.2000.tb06368.x
Vinters HV, Secor DL, Read SL et al (1994) Microvasculature in brain biopsy specimens from patients with Alzheimer’s disease: an immunohistochemical and ultrastructural study. Ultrastruct Pathol 18:333–348. doi:10.3109/01913129409023202
von Arnim CA, Kinoshita A, Peltan ID et al (2005) The low density lipoprotein receptor-related protein (LRP) is a novel beta-secretase (BACE1) substrate. J Biol Chem 280:17777–17785. doi:10.1074/jbc.M414248200
von Tell D, Armulik A, Betsholtz C (2006) Pericytes and vascular stability. Exp Cell Res 312:623–629. doi:10.1016/j.yexcr.2005.10.019
Wang D, Chang PS, Wang Z et al (2001) Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105:851–862. doi:10.1016/S0092-8674(01)00404-4
Wegiel J, Wisniewski HM (1990) The complex of microglial cells and amyloid star in three-dimensional reconstruction. Acta Neuropathol 81:116–124. doi:10.1007/BF00334499
Weller RO, Djuanda E, Yow HY, Carare RO (2009) Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 117:1–14. doi:10.1007/s00401-008-0457-0
Wisniewski T, Frangione B (1992) Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett 135:235–238. doi:10.1016/0304-3940(92)90444-C
Wu Z, Guo H, Chow N et al (2005) Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med 11:959–965
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709. doi:10.1038/sj.jcbfm.9600440
Zhang ET, Inman CB, Weller RO (1990) Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat 170:111–123
Zhong Z, Deane R, Ali Z et al (2008) ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci 11:420–422. doi:10.1038/nn2073
Zlokovic BV (1996) Cerebrovascular transport of Alzheimer’s amyloid beta and apolipoproteins J and E: possible anti-amyloidogenic role of the blood–brain barrier. Life Sci 59:1483–1497. doi:10.1016/0024-3205(96)00310-4
Zlokovic BV (2005) Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci 28:202–208. doi:10.1016/j.tins.2005.02.001
Zlokovic BV (2008) The blood–brain barrier in health and chronic neurodegenerative disorders. Neuron 57:178–201. doi:10.1016/j.neuron.2008.01.003
Zlokovic BV, Yamada S, Holtzman D, Ghiso J, Frangione B (2000) Clearance of amyloid beta-peptide from brain: transport or metabolism? Nat Med 6:718. doi:10.1038/77397
Acknowledgments
This work was supported by the United States National Institutes of Health grants R37AG023084 and R37NS34467 to B.V.Z.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bell, R.D., Zlokovic, B.V. Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta Neuropathol 118, 103–113 (2009). https://doi.org/10.1007/s00401-009-0522-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0522-3