Abstract
Rationale and objectives
In the weaning of patients from mechanical ventilation by gradually reducing pressure support ventilation (PSV), an automated computerized system recently proved to be superior to traditional physician-directed weaning. The aim of this study was to replicate these findings when weaning a broad surgical intensive care unit (ICU) patient population off the ventilator.
Methods and measurements
Sixty patients requiring mechanical ventilation over 24 h were randomized to either automated (n = 30) or physician-directed (n = 30) weaning. The primary endpoint was duration of weaning. Secondary endpoints were duration of mechanical ventilation, length of ICU stay, reintubation rates, and workload for staff.
Results
Weaning duration did not differ significantly between the computer-driven group and the physician-directed group (0.64 vs. 2.33 d, 95%CI: -0.10 to 2.15, p = 0.167). No significant differences were detected for any secondary endpoint except the workload for PSV settings, which was lower in the computer-driven weaning group (0.0 vs. 0.15 settings/h, p < 0.0001). The trial was stopped early because sample size recalculations based on a Pocock design showed it would be pointless to continue.
Conclusions
Computer-driven weaning was not different from traditional physician-directed weaning from mechanical ventilation. Therefore, it cannot be recommended for routine use in a broad surgical ICU patient population.
Zusammenfassung
Begründung und Zielsetzung
Ein automatisiertes Entwöhnungssystem erwies sich kürzlich gegenüber herkömmlicher arztgesteuerter Entwöhnung vom Beatmungsgerät überlegen. Beide Verfahren basierten auf der schrittweisen Reduktion von druckunterstützter Beatmung („pressure support ventilation“). Durch die vorliegende Studie sollte die Wiederholbarkeit dieses Ergebnisses an einem breiten chirurgischen Intensivpatientenkollektiv überprüft werden.
Methoden
Es wurden 60 Patienten mit einer vorhergehenden kontrollierten Beatmungsdauer von mindestens 24 h randomisiert. Jeweils 30 Patienten wurden automatisiert und arztgesteuert entwöhnt. Primärer Endpunkt war die Entwöhnungsdauer. Sekundäre Endpunkte waren die Dauer der mechanischen Beatmung, die Intensivaufenthaltsdauer, die Reintubationsrate und die Arbeitsbelastung des medizinischen Personals.
Ergebnisse
Die Entwöhnungsdauer unterschied sich nicht signifikant zwischen dem automatisiert und arztgesteuert entwöhnten Patientenkollektiv (0,64 vs. 2,33 Tage, 95%-KI: –0,10–2,15, p=0,167). Auch bei den sekundären Endpunkten konnte kein signifikanter Unterschied beobachtet werden. Eine Ausnahme stellte hier die Arbeitsbelastung bei den PSV-Einstellungen dar, die in der computergesteuerten Gruppe signifikant geringer war (0,0 vs. 0,15 Einstellungen pro Stunde, p<0,0001). Die Studie wurde vorzeitig gestoppt, da auf einem Pocock-Design basierende Rekalkulationen zeigten, dass eine Fortführung sinnlos wäre.
Schlussfolgerung
Automatisierte Entwöhnung unterschied sich in den wesentlichen Endpunkten nicht signifikant von konventionell arztgesteuerter Entwöhnung und kann daher nicht generell empfohlen werden.
Similar content being viewed by others
Introduction
Long-term mechanical ventilation is associated with many complications, such as ventilator-associated pneumonia [2], ventilator-induced lung injury [23], respiratory muscle fatigue, and remodeling [17]. Weaning patients from mechanical ventilation as soon as possible is therefore a key issue to avoid clinical complications.
Different approaches have been undertaken to shorten the time on the ventilator, and guidelines for ventilator weaning have been developed [20] but are still the subject of controversy. In this context, automated computerized systems have recently become important. Automated weaning (AW) is expected to expedite the weaning process compared to conventional physician-directed weaning (PDW). Apart from potential benefits for patients, there might be benefits for ICU physicians in terms of reduced workload.
A computerized automated system, based on stepwise reduction of pressure support ventilation (PSV), was developed by Dojat et al. [6, 9]. Further details on this system, which is now commercially available as SmartCareTM/PS (Dräger Medical, Lübeck, Germany) have been described elsewhere [5, 6, 7, 8, 9].
A randomized multicenter trial by Lellouche et al. [19] showed that this system significantly reduces duration of weaning, duration of mechanical ventilation, and length of stay in the ICU for a given patient when compared with protocolized PDW. PDW was also conducted by gradually reducing pressure support ventilation (PSV).
In daily routine, initiating protocolized PDW is complex due to many reasons [10]. Accordingly on surgical ICUs, protocol adherence has been estimated to be only 63% [11]. Thus, the automated system by Dojat et al. seems to have advantages over a physician-directed protocol.
Bearing these facts in mind, we tested the computerized automated system using SmartCareTM/PS versus non-protocolized physician-directed weaning, which more realistically parallels conditions in surgical ICUs.
The aim of this study was to replicate the findings by Lellouche et al. in a surgical ICU population which differed broadly in terms of age and prior duration of mechanical ventilation. In contrast to the previous study, the staff workload was estimated in both study arms.
Methods
Patients
Patients were recruited in the surgical ICU after receiving consent from the local ethics committee. Before inclusion, signed informed consent was obtained. After having been mechanically ventilated via endotracheal tube or tracheostomy for at least 24 h, patients were included when breathing spontaneously at a Ramsay sedation score ≤3. Other inclusion criteria were sufficient arterial oxygenation with paO2 >75 cm H2O or SaO2 >90% at FiO2 of no more than 0.5. Patient age had to be between 18 and 80 years, body weight between 35 kg and 200 kg. Exclusion criteria were a PEEP level >10 cm H2O, hemodynamic instability with a high demand for catecholamines (i.e., >5 µg/kg/min dopamine), rectal body temperature >39°C, hemoglobin concentration <7 g/dl and pH <7.2. Patients were not blinded.
Interventions
Patients fulfilling all inclusion criteria were randomized to either AW or to PDW. Stratified randomization with grouped variables of age (≤60 and >60 years) and duration of mechanical ventilation before initiation of weaning (1–2 d, 3–10 d, >10 d) was performed using opaque sealed envelopes. Within strata, permuted block randomization was chosen with a block size of four.
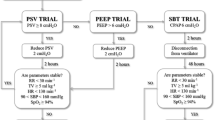
In both study arms, the EvitaXL ventilator (Dräger Medical, Lübeck, Germany) was used. Basic weaning conditions for all patients were usage of the EvitaXL-CPAP (continuous positive airway pressure)/ASB (assisted spontaneous breathing; equivalent to PSV) mode, active humidification of inspired gas, activated monitoring of end-tidal CO2 concentration and no night’s rest. Specifically, weaning was conducted using the embedded protocol described above in the SmartCare/PS group, whereas in the physician-directed group no strict protocol was demanded. The sole requirement here was that PSV should be gradually reduced with single steps of no more than 10 cm H2O.
Abortion of ventilator weaning was carried out when controlled ventilation was needed again, e.g., due to lactate acidosis or FiO2 of more than 0.5. The study protocol allowed one abortion and two weaning trials for each participant, respectively.
Extubation (in the case of intubation) or disconnection (in the case of tracheostomy) was carried out if the following extubation criteria were met: respiratory rate <30/min, paO2 >75 cm H2O or SaO2 >90%, sufficient airway protection and hemodynamic stability. After extubation, patients were followed up for 48 h, and extubation was classed a success in those cases where there was no need for reintubation within 48 h.
Endpoints
The primary endpoint was the duration of ventilator weaning, defined as the time from inclusion until extubation or disconnection.
Secondary endpoints were the total duration of mechanical ventilation until extubation, length of ICU stay and complications in both study arms in terms of reintubation within 48 h after extubation. In addition, the workload was investigated separately for physicians and nurses. For physicians, workload was measured by the quantity of PSV, FiO2, and PEEP settings per hour. For nurses, the frequency of the alarm “Clean CO2 cuvette” per hour was recorded, indicating cleaning demand and, thus, workload when using active humidification of inspired gas.
Mortality was compared between both study groups in terms of ICU and hospital mortality.
Sample size calculation and sample size re-calculation
The primary endpoint was weaning time (days). With a mean weaning time of 3 d in the computerized automated weaning group and a mean weaning time of 5 d in the physician-directed weaning group, the sample size required to achieve 80% power at a significance level of 5% and a drop-out rate of 5% based on the two-sided t-test was 54 patients per treatment group. The study was planned to be completed after a 1-year recruitment phase.
Since the recruitment process was delayed, however, only 50 patients were included in the trial after one year instead of the 108 planned. Since mentoring was limited to 1.5 years, the study design was changed. Specifically, the local ethics committee was informed about an interim analysis, which was previously not planned. For adaptation of the study design, the conditional error function principle of Müller and Schäfer was adopted [21]. The interim analysis was performed based on a group sequential plan with one interim analysis using the α-spending function according to Pocock. The interim analysis was carried out after enrollment of 60 patients, and the sample size was re-calculated.
Statistical analysis
The exact two-sided Mann-Whitney U test was used for testing the primary hypothesis, and Hodges-Lehmann estimates together with exact 95% confidence intervals (95% CI) were computed. Analyses were performed using the intention-to-treat principle. If the primary endpoint could not be assessed because of any kind of complication or death, the primary endpoint was set to the worst possible value, i.e., the maximum weaning time observed. Complete case analyses were carried out as sensitivity analyses. All other analyses were considered exploratory.
Differences in reintubation frequencies within 48 h were investigated using the Fisher’s exact two-sided test. Total mechanical ventilation time and total length of stay in the ICU were compared using the exact two-sided Mann-Whitney U test. Appropriate 95% confidence intervals were added.
Results
Patients
Sixty patients were enrolled in the study after testing of 479 patients for eligibility from November 2, 2005 to January 21, 2007. Thirty patients were randomly allocated to the computerized and thirty patients to the physician-directed weaning group.
Baseline patient characteristics are displayed in Tab. 1. Participants were similar in both study groups, especially in terms of duration of mechanical ventilation before weaning, lung function, and disease severity.
Twenty-six of 30 patients within each study arm (n=52) could be extubated after a maximum of two weaning trials and were followed up for 48 h (Fig. 1) for endpoint evaluation. Thirty-eight of these 52 patients were successfully extubated, whereas 14 (n = 8 in AW; n = 6 in PDW) patients had to be reintubated within the follow-up period of 48 h, and they were classified as therapy failures. The remaining 8 patients could not be extubated under study conditions and were classified as drop-outs.
Outcome
The primary endpoint did not differ significantly between the two study arms when all patients were analyzed (medians AW: 0.64 and PDW: 2.33; 95%CI for median difference: –0.10 to 2.15; p=0.167) (Tab. 2).
Results for secondary endpoints are summarized in Tab. 3. Only one significant difference could be identified: PSV settings, as part of the weaning workload for physicians, were significantly reduced in AW (median 0.00) versus PDW (median 0.15; 95% CI for median difference: 0.11–0.18; p<0.0001). On the other hand FiO2 and PEEP settings were similar in both study arms. The workload for nurses, indirectly indicated via the alarm “Clean CO2 cuvette” per hour, showed a cleaning demand every ~1.5 h in both groups.
All other secondary outcomes did not differ significantly between study groups (Tab. 3).
Ten of the 60 study participants died during their stay in the ICU or in the hospital, resulting in a hospital mortality of 16.7%. Mortality showed similar results when comparing both groups (Tab. 3).
Sample size re-calculation and stopping due to futility
With a one-sided p value of 0.0835 observed first stage, a one-sided p value of 0.0373 would be required in the second stage in order to claim significance at a global two-sided test level of 0.05. Assuming a constant effect size of 0.167 in the interim analysis, the stage two sample size would have been 522 per group, i.e., 1044 in total given a global significance level of 5% at 80% power.
The study was, therefore, stopped after the enrollment of 60 patients, since it was considered pointless to continue. The local ethics committee was informed on May 31, 2007.
Discussion
Unlike the results found by Lellouche et al. [19], no significant difference was observed in the interim analysis. Given the effect that was observed, another 1044 patients would have to be enrolled in a second stage, and the study was stopped because it was considered futile. The fundamental question is why the study results are different although study designs were similar. Comparing patient characteristics between studies, the following differences were observed: patients in the AW and PDW study groups were approximately 5 years older in this study (median 66 and 67 years) compared to the patients included by Lellouche et al. (median 60 and 62 years). Furthermore, the duration of mechanical ventilation before inclusion was 1.4 d longer in our study (5.4 vs. 5.9 d compared with 3.5 vs. 4.0 d), whereas disease severity was higher in the Lellouche study (38.0 vs. 39.0 compared with 49.0 vs. 47.5). Unfortunately, lung function, reflected by the oxygenation index, cannot be compared because no data were reported by Lellouche et al. Also two-thirds of their patients were admitted to the ICU for medical reasons and one-third for surgical reasons. In our study, patients were admitted only for surgical reasons. In conclusion, heterogeneity between the study populations might explain the difference, at least in part.
Nevertheless, this study ascertained that complication rates, as described previously, were similar between groups. Reintubation rates in our study (30.1 vs. 23.1%) were admittedly higher than those found in the Lellouche study (23.0 vs. 16.0%) and those reported in the literature (4–23%) [1, 13, 14, 24]. This should be alarming since reintubation is associated with higher mortality. But mortality in our study was low (hospital mortality: 16.7 vs. 16.7%; ICU mortality: 10.0 vs. 6.7%), as can be generally expected for surgical ICUs [4, 12, 15] and was lower than in the Lellouche study (hospital mortality: 37.8 vs. 28.6%; ICU mortality: 21.6 vs. 22.9%). One possible reason for higher reintubation rates in our study is the inclusion of tracheotomized patients. After disconnection from the ventilator, the tracheal tube remained in place and could be easily reconnected to the ventilator when needed. Willingness to reconnecting a tracheotomized patient to the ventilator can be assumed to be higher than reintubating a non-tracheotomized patient, where other procedures such as non-invasive ventilation were ruled out beforehand.
Furthermore, we assumed the workload would be lower when using a computerized automated system than when using traditional manual weaning. But the analysis showed a reduced workload only in terms of PSV settings per hour. No general decrease was found. On the contrary, a higher workload has to be assumed when using active humidification of inspired gas, because the end-tidal CO2 measurements regularly fog up and have to be cleaned every 1.5 h by nurses. Under these circumstances, acceptance of automation by personnel was poor.
In this context, it has to be mentioned that the cleaning workload can be reduced dramatically by using passive methods to moisten inspired gas, for instance, a heat and moisture exchanger (HME). Active and passive techniques for humidification of inspired gas have advantages and disadvantages. Neither was able to reduce the incidence of ventilator-associated pneumonia, which explains why no unequivocal recommendation can be given to date [3, 16, 22]. In terms of workload for nurses, we recommend the use of passive techniques for humidification when using automated weaning.
Finally we wish to point out the limitations of our study:
-
1.
Weaning conditions in the physician-directed weaning group were intentionally chosen to be imprecise in order to examine native physician behavior. This might result in bias since ventilator settings per hour might have been higher or lower than usual.
-
2.
Inclusion of intubated as well as tracheotomized patients is problematic since both groups are often different, especially in terms of disease severity. And heterogeneity of the study population means less comparability. On the other hand, including both groups displays ICUs daily routine more realistically.
-
3.
The study population did not include patients mechanically ventilated for less than 24 h. The value of both methods within this subpopulation remains unclear.
-
4.
Most of the patients included (65%) were ventilated over 5 d before initialization of weaning. This does not correspond to normal conditions in ICUs. For instance, the results of a German ICU register [18] showed an average duration of mechanical ventilation of 3.2 d for survivors and 8.0 d for deaths.
-
5.
The duration of weaning measured in both arms of our study (0.64 vs. 2.33 d) differed clearly from the results measured by Lellouche et al. (3 vs. 5 d). In both studies, weaning was started when patients were ready for spontaneous assisted breathing. Obviously weaning started later in our study, namely when sedation was low (Ramsay score <3). However, the degree of sedation is to some extent a subjective judgment. It is likely that physicians waited longer when they were not certain about the alertness of a given patient. Nevertheless, the total duration of ventilation was lower in both study arms of our study (5.65 vs. 8.31 d) than in the Lellouche study (12 vs. 7.5 d).
Conclusions
In all relevant endpoints no significant difference was observed between automated and physician-directed weaning. Therefore, AW cannot be recommended for weaning a broad surgical intensive care population in general.
Literatur
Brochard L, Rauss A, Benito S et al (1994) Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med 150(4):896–903
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165(7):867–903
Davis K Jr, Evans SL, Campbell RS et al (2000) Prolonged use of heat and moisture exchangers does not affect device efficiency or frequency rate of nosocomial pneumonia. Crit Care Med 28(5):1412–1418
de Rooij SE, Govers A, Korevaar JC et al (2006) Short-term and long-term mortality in very elderly patients admitted to an intensive care unit. Intensive Care Med 32(7):1039–1044
Dojat M, Brochard L (2001) Knowledge-based systems for automatic ventilatory management. Respir Care Clin N Am 7(3):379–396, viii
Dojat M, Brochard L, Lemaire F, Harf A (1992) A knowledge-based system for assisted ventilation of patients in intensive care units. Int J Clin Monit Comput 9(4):239–250
Dojat M, Harf A, Touchard D et al (1996) Evaluation of a knowledge-based system providing ventilatory management and decision for extubation. Am J Respir Crit Care Med 153(3):997–1004
Dojat M, Harf A, Touchard D et al (2000) Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med 161(4 Pt 1):1161–1166
Dojat M, Pachet F, Guessoum Z et al (1997) NeoGanesh: a working system for the automated control of assisted ventilation in ICUs. Artif Intell Med 11(2):97–117
Ely EW, Bennett PA, Bowton DL et al (1999) Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med 159(2):439–446
Ely EW, Bennett PA, Bowton DL et al (1999) Large scale implementation of a respiratory therapist-driven protocol for ventilator weaning. Am J Respir Crit Care Med 159(2):439–446
Engel JM, Junger A, Bottger S et al (2003) Outcome prediction in a surgical ICU using automatically calculated SAPS II scores. Anaesth Intensive Care 31(5):548–554
Epstein SK, Ciubotaru RL (1998) Independent effects of etiology of failure and time to reintubation on outcome for patients failing extubation. Am J Respir Crit Care Med 158(2):489–493
Esteban A, Alia I, Gordo F et al (1997) Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 156(2 Pt 1):459–465
Fischler L, Lelais F, Young J et al (2007) Assessment of three different mortality prediction models in four well-defined critical care patient groups at two points in time: a prospective cohort study. Eur J Anaesthesiol 24(8):676–683
Girault C, Breton L, Richard JC et al (2003) Mechanical effects of airway humidification devices in difficult to wean patients. Crit Care Med 31(5):1306–1311
Le BG, Viires N, Boczkowski J et al (1994) Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med 149(6):1539–1544
Lefering R (2002) für die Interdisziplinäre Arbeitsgruppe “Qualitätssicherung in der Intensivmedizin” der Deutschen Interdisziplinären Vereinigung für Intensivmedizin (DIVI). Erste Ergebnisse des nationalen Registers zum externen Qualitätsvergleich der Intensivmedizin. Intensivmed 39(4):334–340
Lellouche F, Mancebo J, Jolliet P et al (2006) A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med 174(8):894–900
MacIntyre NR, Cook DJ, Ely EW Jr et al (2001) Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 120 (6 Suppl):375S–395S
Müller HH, Schäfer H (2004) A general statistical principle for changing a design any time during the course of a trial. Stat Med 23:2497–2508
Ricard JD, Boyer A, Dreyfuss D (2006) The effect of humidification on the incidence of ventilator-associated pneumonia. Respir Care Clin N Am 12(2):263–273
Slutsky AS (1999) Lung injury caused by mechanical ventilation. Chest 116 (1 Suppl):9S–15S
Vallverdu I, Calaf N, Subirana M et al (1998) Clinical characteristics, respiratory functional parameters and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med 158 (6):1855–1862
Conflict of interest
The corresponding author states that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stahl, C., Dahmen, G., Ziegler, A. et al. Comparison of automated protocol-based versus non-protocol-based physician-directed weaning from mechanical ventilation. Intensivmed 46, 441–446 (2009). https://doi.org/10.1007/s00390-009-0061-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00390-009-0061-0