Abstract
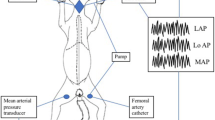
Endothelium-derived relaxing factor (EDRF) or nitric oxide (NO) biosynthesis fromL-arginine occurs in the endothelium and platelets and may modulate plate-let function and contribute to thromboresistance in the vessel wall. A rat model was used to evaluate selective accumulation of111In-labeled platelets in the pulmonary microcirculation following the administration of collagen, adenosine 5′-diphosphate (ADP) or thrombin. Platelet aggregation was monitored continuously over the thorax using a microcomputer-based system. Sodium nitroprusside, a stimulator of soluble guanylate cyclase and zaprinast, a phosphodiesterase V inhibitor, both known to cause accumulation of cyclic guanosine monophosphate, exhibited moderate inhibitory activity, which was shared byL-arginine. NG-monomethyl-L-arginine (L-NMMA; 1 mg/kg/min), an inhibitor of EDRF(NO), potentiated the aggregatory response to collagen at an intravenous dose of 100 µg/kg but not at one of 30 µg/kg. D-NMMA had no such effect. The augmenting effect of L-NMMA was abolished byL-arginine. NG-nitro-L-arginine methyl ester (L-NAME; 0.1 mg/kg/min) also markedly augmented the collagen-induced platelet response, and, at higher doses, all treated animals died upon collagen challenge. Both L-NMMA and L-NAME did not affect the responses to ADP and thrombin. The results suggest that in the intact vascular system, basal release of EDRF(NO) is not critically involved in modulation of platelet function but becomes a significant factor when platelets are exposed to great amounts of collagen fibrils.
Similar content being viewed by others
References
Alheid U, Frolich JC, Forstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res 47:561–571;1987.
Azuma H, Ishikawa M, Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol 88:411–415;1986.
Bhardwaj R, Page CP, May GR, Moore PK. Endothelium-derived relaxing factor inhibits platelet aggregation in human whole blood in vitro and in the rat in vivo. Eur J Pharmacol 157:83–91;1988.
Bohme E, Jung R, Mechler I. Guanylate cyclase in human platelets. In: Hardman JG, O'Malley BW, eds. Methods in Enzymology, vol 38. New York, Academic Press, 199–202;1974.
Busse R, Luckhoff A, Bassenge E. Endothelium-derived relaxant factor inhibits platelet activation. Naunyn-Schmiedebergs Arch Pharmacol 336:566–571;1987.
DiMinno G, Silver MJ. Mouse antithrombotic assay: A simple method for the evaluation of antithrombotic agents in vivo. Potentiation of antithrombotic activity by ethyl alcohol. J Pharmacol Exp Ther 225:57–60;1983.
Dusting GJ, MacDonald PS. Prostacyclin and vascular function: Implications for hypertension and atherosclerosis. Pharmacol Ther 48:323–344;1990.
Furlong B, Henderson AH, Lewis MJ, Smith JA. Endothelium-derived relaxant factor inhibits in vitro platelet aggregation. Br J Pharmacol 90:687–692;1987.
Harris AL, Lemp BM, Bentley RG, Perrone MH, Hamel LT, Silver PJ. Phosphodiesterase isozyme inhibition and the potentiation by zaprinast of endothelium-derived relaxing factor and guanylate cyclase stimulating agents in vascular smooth muscle. J Pharmacol Exp Ther 249:394–400;1989.
Hogan JC, Lewis MJ, Henderson AH. In vivo EDRF activity influences platelet function. Br J Pharmacol 94:1020–1022;1988.
Klee A, Seiffge D. Evaluation of pulmonary accumulation of51chromium-labelled rat platelets following intravenous application of ADP and collagen. Thromb Haemost 65:588–595;1991.
May GR, Crook P, Moore PK, Page CP. The role of nitric oxide as an endogenous regulator of platelet and neutrophil activation within the pulmonary circulation of the rabbit. Br J Pharmacol 102:759–763;1991.
Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142;1991.
Mugge A, Forstermann U, Lichtlen PR. Platelets, endothelium-dependent responses and atherosclerosis. Ann Med 23:545–550;1991.
Nolte C, Eigenthaler M, Schanzenbacher P, Walter U. Endothelial cell-dependent phosphorylation of a platelet protein mediated by cAMP- and cGMP-elevating factors. J Biol Chem 266:14808–14812;1991.
Oyekan AO, Botting JH. Indium-111 oxine technique of studying platelet aggregation in vivo. Acta Haematol 83:57–64;1990.
Radomski MW, Palmer RMJ, Moncada S. The anti-aggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. Br J Pharmacol 92:639–646;1987.
Radomski MW, Palmer RMJ, Moncada S. Characterization of the L-arginine: nitric oxide pathway in human platelets. Br J Pharmacol 101:325–328;1990.
Rees DD, Palmer RMJ, Schulz R, Hodson HF, Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol 101:746–752;1990.
Smith D, Sanjar S, Herd C, Morley J. In vivo method for the assessment of platelet accumulation. J Pharmacol Methods 21:45–59;1989.
Stamler JS, Loscalzo J. The antiplatelet effects of organic nitrates and related nitroso compounds in vitro and in vivo and their relevance to cardiovascular disorders. J Am Coll Cardiol 18:1529–1536;1991.
Venturini CM, Del Vecchio PJ, Kaplan JE. Thrombin induced platelet adhesion to endothelium is modified by endothelial derived relaxing factor (EDRF). Biochem Biophys Res Commun 159:349–354;1989.
Weishaar RE, Burrows SD, Lobylarz DC, Quade MM, Evans DB. Multiple molecular forms of cyclic nucleotide phosphodiesterase in cardiac and smooth muscle and in platelets: Isolation, characterization and effects of various reference phosphodiesterase inhibitors and cardiotonic agents. Biochem Pharmacol 35:787–800;1986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chiu, P.J.S., Tetzloff, G. EDRF(NO)-mediated modulation of collagen-induced platelet accumulation in rat pulmonary microcirculation. J Biomed Sci 1, 43–48 (1993). https://doi.org/10.1007/BF02258338
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02258338